| Could 24, 2023 |
|
(Nanowerk Information) Designing the subsequent technology of environment friendly vitality conversion units for powering our electronics and heating our properties requires an in depth understanding of how molecules transfer and vibrate whereas present process light-induced chemical reactions. Researchers on the Division of Power’s Lawrence Berkeley Nationwide Laboratory (Berkeley Lab) have now visualized the distortions of chemical bonds in a methane molecule after it absorbs gentle, loses an electron, after which relaxes. Their examine gives insights into how molecules react to gentle, which might finally be helpful for creating new strategies to manage chemical reactions.
|
|
Analyzing how a molecule responds to gentle on extraordinarily quick timescales permits researchers to trace how electrons transfer throughout a chemical response. “The large query is how a molecule dissipates vitality with out breaking up,” mentioned Enrico Ridente, a physicist at Berkeley Lab and lead creator on the Science paper reporting the work (“Femtosecond symmetry breaking and coherent leisure of methane cations through x-ray spectroscopy”). This implies inspecting how extra vitality is redistributed in a molecule that has been excited by gentle, because the electrons and nuclei transfer about whereas the molecule relaxes to an equilibrium state.
|
|
Probing these fine-scale actions means making observations of processes that happen on timescales quicker than a millionth of a billionth of a second. For many years, researchers have relied on principle to explain how extra vitality impacts the symmetry of – however doesn’t break – the bonds of a molecule that’s been excited by gentle. This principle predicts how the bond lengths and angles between particular person atoms ought to change whereas electrons shift place, and what intermediate buildings it ought to undertake.
|
|
Now, utilizing ultrafast x-ray spectroscopy services at Berkeley Lab’s Chemical Sciences Division, Ridente and his colleagues noticed how the construction of ionized methane molecules evolves over time.
|
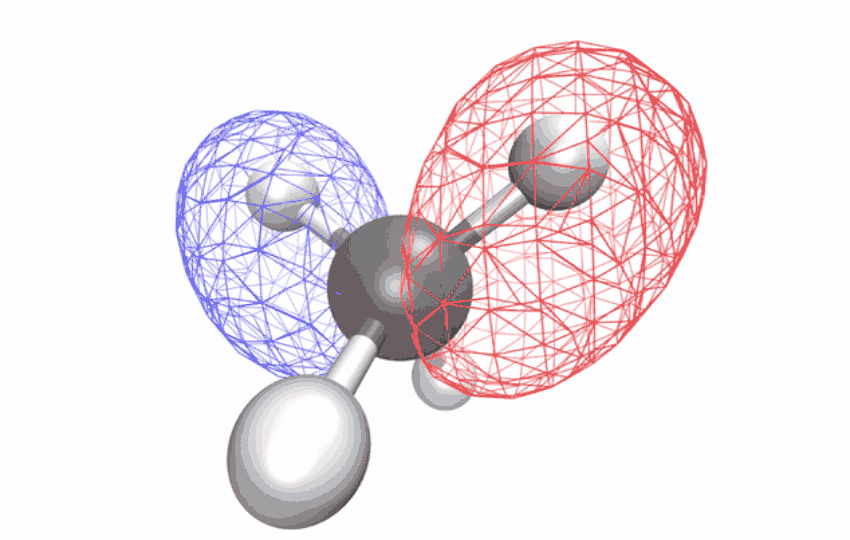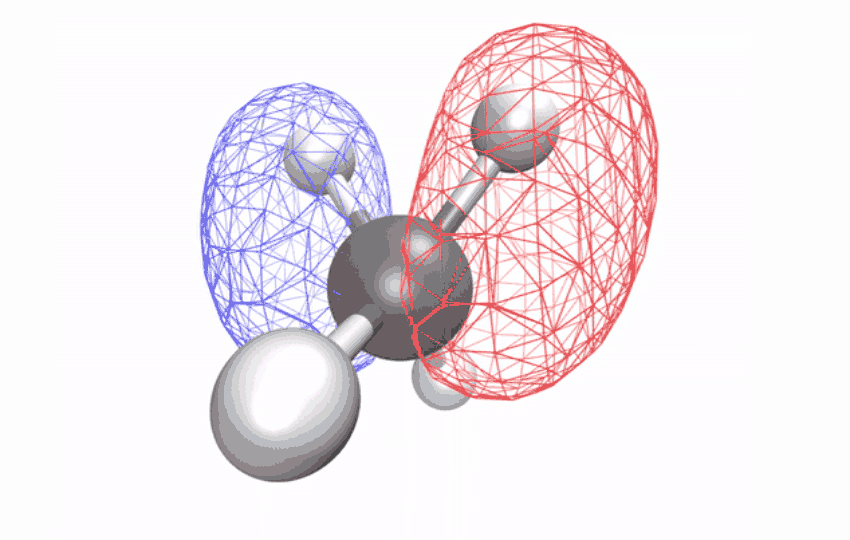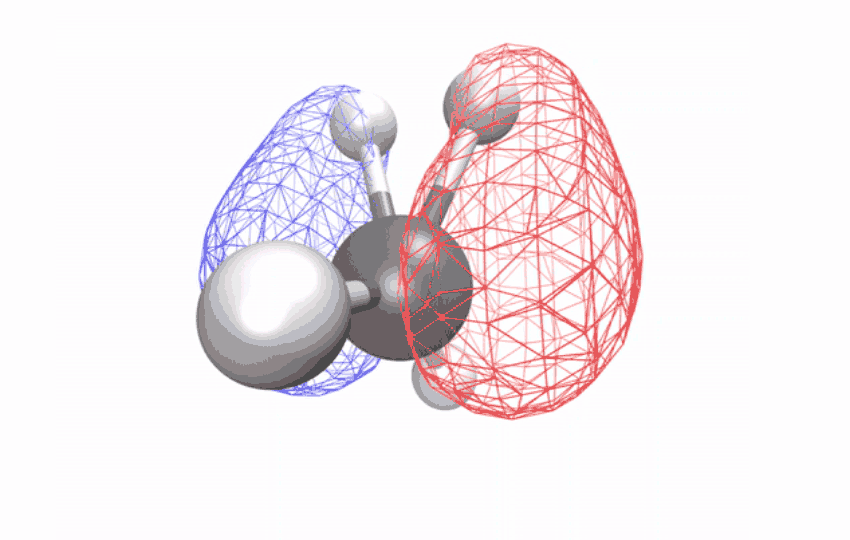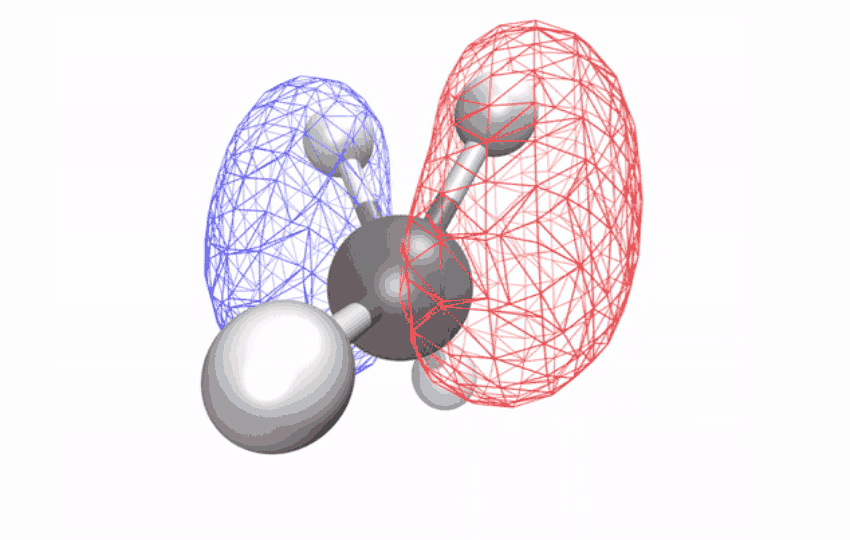 |
| The angles between atoms in an excited methane molecule change because the molecule relaxes, distorting its form and redistributing the absorbed vitality. (Picture: Diptarka Hait/Berkeley Lab)
|
|
“Methane ions are a super system to handle this query as a result of they don’t come aside when excited by gentle,” mentioned Ridente.
|
|
By first utilizing a laser to strip an electron from the impartial methane molecule, then taking ultrafast x-ray spectroscopic snapshots of the remaining ion, the researchers collected a time sequence of spectral alerts. The alerts revealed how the initially symmetric form turns into distorted over a ten-femtosecond interval (a femtosecond is one quadrillionth of a second) – observational proof of a long-studied impact referred to as Jahn-Teller distortion. Longer time observations confirmed that for an additional 58 femtoseconds, the distorted form vibrates coherently in a scissoring-like movement whereas redistributing its vitality through different vibrations via the construction’s geometric modifications.
|
|
“Thanks to those measurements and the understanding gained from principle, we have been in a position to time-resolve the total evolution of the distortion for the primary time,” mentioned Stephen Leone, a chemist at Berkeley Lab and the senior creator on the Science paper.
|
|
The researchers used the Cori and Perlmutter techniques on the Nationwide Power Analysis Scientific Computing Heart (NERSC), a DOE Workplace of Science consumer facility at Berkeley Lab, to carry out calculations that confirmed their measurements of the molecule’s actions.
|
|
“We are able to now clarify how the molecule distorts after shedding an electron and the way the energies of the electrons reply to those modifications,” mentioned Diptarka Hait, a graduate pupil at Berkeley Lab and the lead theoretical creator of the examine.
|
|
The examine demonstrated the viability of an x-ray method for learning ultrafast molecular dynamics. Methane is a elementary but easy molecule the place one of the primary kinds of distortions happens as predicted, however with richer and extra difficult dynamics than beforehand understood. “This analysis opens the door for learning extra advanced techniques and different kinds of distortions,” says Ridente. Such insights in regards to the dynamics of electrons and nuclei can result in improvements in new vitality conversion units and photocatalysis purposes.
|



