Xin-Wen Zhou, from the School of Supplies and Chemical Engineering at China Three Gorges College, spearheaded new analysis that explores a simple methodology to synthesize a variety of PdFe/Cu catalysts by way of a step-by-step discount course of involving floor reconstruction.
The ethanol oxidation response (EOR) serves as a vital anode response in direct ethanol gasoline cells (DEFCs), historically counting on the noble steel platinum (Pt) as its very best anode catalyst.
Nevertheless, the excessive value, restricted applicability, and inadequate resistance to CO poisoning hinder the widespread adoption of this method. Latest analysis signifies that synthesizing new catalysts by combining valuable metals with non-noble metals and non-metals successfully reduces prices and enhances catalyst efficiency.
Morphology management, floor engineering, improved provider results, and even the adoption of a single-atomic technique present promise in considerably boosting the utilization of valuable metals.
Research more and more exhibit that the electrooxidation of ethanol in alkaline media not solely displays sooner kinetics but additionally boasts excessive exercise and long-term stability. Within the alkaline system, the usage of the cheaper Pd (in comparison with Pt) because the lively materials for catalytic ethanol oxidation has been explored.
Analysis means that the formation of noble metal-transition steel alloy catalysts (for instance, PdFe, PdNi, PdAu, PdCo, PdAg, PdCu, and AlNiCuPtPdCo) or noble steel/nonmetal composite supplies not solely reduces the Pd content material within the catalyst but additionally performs a pivotal function in enhancing the electrooxidation efficiency of ethanol.
Lately, developments in anionic strong electrolyte membranes have considerably propelled analysis on direct alkaline ethanol gasoline cells (DAEFCs), with industrial functions on the horizon.
The elevated kinetics of ethanol response in an alkaline answer will be attributed to the pH-induced detrimental shift within the working potential of 59 mV/pH, altering the native electrical double layer construction and electrical subject distribution on the electrode/electrolyte interface, thereby enhancing electrocatalytic exercise.
In an alkaline setting, the soundness of lively transition metals (for instance, Fe, Ni, Co, Cu) will be enhanced, significantly by forming a multi-metal Pd-based catalyst. Introducing Cu2+ ions throughout precursor levels transforms spherical PtFe nanoparticles into PtFeCu nanochains, showcasing enhanced methanol oxidation properties.
PdFe-based catalysts, famous for his or her excessive electrical conductivity, distinctive catalytic exercise, and sturdiness, are regularly employed as electrocatalysts for the oxygen discount response (ORR) or anode oxidation response. The synergy between the core and shell introduces stress-strain results, altering the floor’s digital construction and influencing oxygen adsorption and discount.
The part transformation of face-center cubic (fcc) to face-center tetragonal (fct) in PdFe@Pd will increase the catalyst’s lively web site, thereby enhancing ORR catalytic efficiency.
The assembled nano PdFe alloy movie serves as a extremely environment friendly electrocatalyst for hydrogen evolution in each acidic and alkaline options as a result of modifications within the valence electron construction of Pd and a rise within the electrochemical lively space (ECSA).
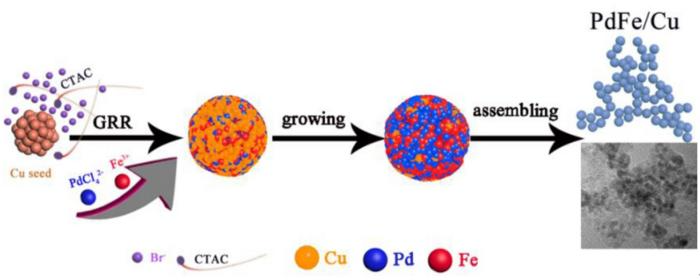
The galvanic alternative response (GRR), an in-situ sacrificial template methodology, is employed for hole nanocatalyst preparation by way of a possible displacement methodology.
The stepwise co-reduction methodology is utilized on this research to design a PdFe/Cu nanocatalyst with a decreased particle measurement. The GRR course of induces a robust reconstituted impact, oxidizing the floor of the Cu seed crystal and ensuing within the formation of superfine PdFe/Cu nanoparticles.
The synergy and strain-stress results amongst Pd, Fe, and Cu, induced by this structural change, alter the digital construction of the nanoparticles, proving advantageous for ethanol molecule adsorption and subsequent oxidation reactions.
Comparative testing with three management catalysts (CuPdFe, PdFe, and CuPdFe/Cu) beneath optimized situations reveals the catalyst with the optimum electrooxidation efficiency of ethanol within the alkaline system.
Journal Reference:
Chen, D., et al. (2023) Extremely lively and sturdy PdFe/Cu nanocatalysts ready by liquid part synthesis for ethanol electrooxidation response. Superior Sensor and Power Supplies. doi.org/10.1016/j.asems.2023.100075.
Supply: https://www.zhongkeqikan.com/


