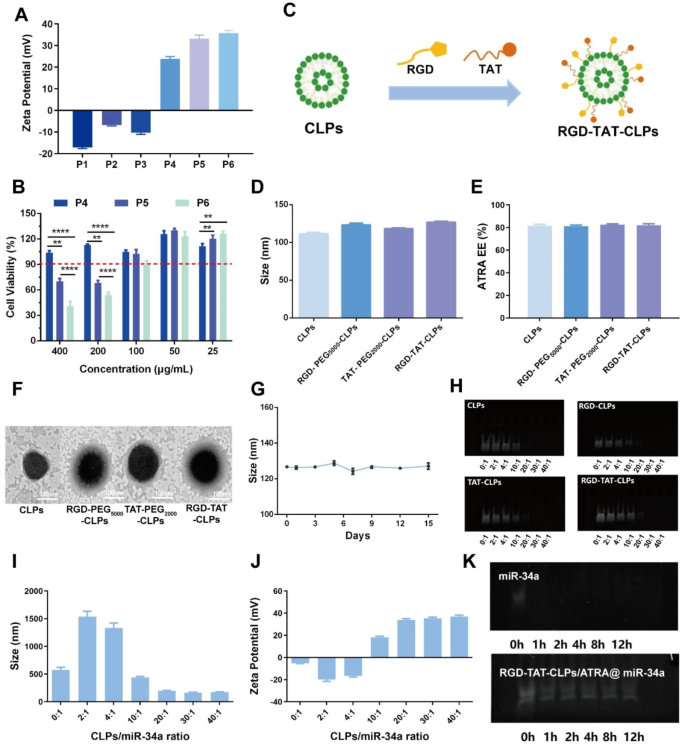
Preparation and characterization of RGD-TAT-CLPs/ATRA@miR-34a
The proposed RGD-TAT-CLPs/ATRA@miR-34a was ready by a skinny layer dispersion methodology. Firstly, a collection of parameters have been optimized to assemble the clean liposomes with superior particle dimension and encapsulation effectivity (EE), together with egg phospholipids (EP)/ldl cholesterol (Chol) ratio, hydration quantity, ultrasonic energy and ultrasonic time (Figs. S1-S4). Then, the cationic lipid (2,3-Dioleoyloxy-propyl)-trimethylammonium-chloride (DOTAP) and helper lipid 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) with completely different including ratios have been concerned within the preparation of CLPs (Desk S1). The obtained CLPs formulations P1-P6 have been subjected to Zeta Potential and security analysis. As proven in Fig. 1A, the cationic floor was proven in P4-P6 however not in P1-P3, indicating that DOTAP content material higher than 3 mg is crucial to assemble CLPs. Nonetheless, the higher the DOTAP contents, the more serious the security for regular cells within the respiratory system. Thus, the impact of CLPs on the conventional human bronchial epithelial cells Base-2b viabilities was decided to judge their security (Fig. 1B). Contemplating that the encapsulated ATRA in 100 µg/mL CLPs may meet the security necessities (Fig. S5), P5 was chosen because the optimized CLPs formulation. Then, the proposed CLPs have been additional modified with RGD peptide and TAT peptide to assemble RGD-TAT-CLPs (Fig. 1C). As proven in Fig. 1D and Figs. S6-S7, the modification of the peptide didn’t clearly change the particle dimension, polydispersity index (PDI) and floor Zeta Potential. No vital change of ATRA EE was noticed as nicely, demonstrating the happy drug loading capability of RGD-TAT-CLPs (Fig. 1E). As proven within the transmission electron microscope (TEM) photographs, an evident shell construction was proven within the peptides-modified CLPs, through which RGD-TAT-CLPs confirmed the thickest shell, in all probability because of the twin peptide modifications (Fig. 1F). Then, the storage stability of RGD-TAT-CLPs was additionally studied primarily based on the particle dimension, PDI and Zeta Potential measurements, which mirrored the right stability inside 15 days (Fig. 1G and Figs. S8-S9).
Having efficiently established the RGD-TAT-CLPs, ATRA and miR-34a have been loaded to assemble the RGD-TAT-CLPs/ATRA@miR-34a. The ATRA encapsulation ratio was optimized as 1:20 in line with the best EE of about 88.37% and desired drug launch profile (Desk S2 and Fig. S10). Then, the miR-34a was adsorbed on the optimized formulation with completely different RGD-TAT-CLPs/ATRA:miR-34a ratios. As proven in Fig. 1H, the unloaded free miR-34a was measured by a gel electrophoresis evaluation. Solely when the ratio was increased than 10:1 may the miR-34a be fully adsorbed, and the formulation of 20:1 may additionally exhibit the specified particle dimension and constructive Zeta Potential (Fig. 1I-1J). Thus, the RGD-TAT-CLPs/ATRA:miR-34a ratio of 20:1 was chosen because the optimized formulation. In comparison with free miR-34a, the steadiness of the encapsulated miR-34a in fetal bovine serum (FBS) was terribly improved (Fig. 1Okay). After incubation of 12 h, the miR-34a may nonetheless preserve stability whereas the free miR-34a was fully degraded inside 1 h. Taken collectively, the RGD-TAT-CLPs/ATRA@miR-34a was efficiently ready, which co-loaded ATRA and miR-34a and improved the steadiness of miR-34a.
Preparation and characterization of RGD-TAT-CLPs/ATRA@miR-34a: (A-B) Zeta Potential (A) and cytotoxicity on Base-2b cells (B) of various CLPs formulations. (C) The schematical illustration of RGD-TAT-CLPs preparation. (D-F) The particle dimension (D), ARTA EE (E) and TEM morphology (F) of CLPs, RGD-PEG5000-CLPs, TAT-PEG2000-CLPs and RGD-TAT-CLPs. (G) The steadiness of RGD-TAT-CLPs. (H) The gel electrophoresis photographs of free miR-34a within the preparation of CLPs@miR-34a with completely different concentrating on peptides and completely different miR-34a loading. (I-J) The particle dimension (I) and Zeta Potential (J) of RGD-TAT-CLPs/ATRA@miR-34a with completely different miR-34a loading. (Okay) The gel electrophoresis photographs of miR-34a and RGD-TAT-CLPs/ATRA@miR-34a below FBS situation inside 12 h. (n = 3)
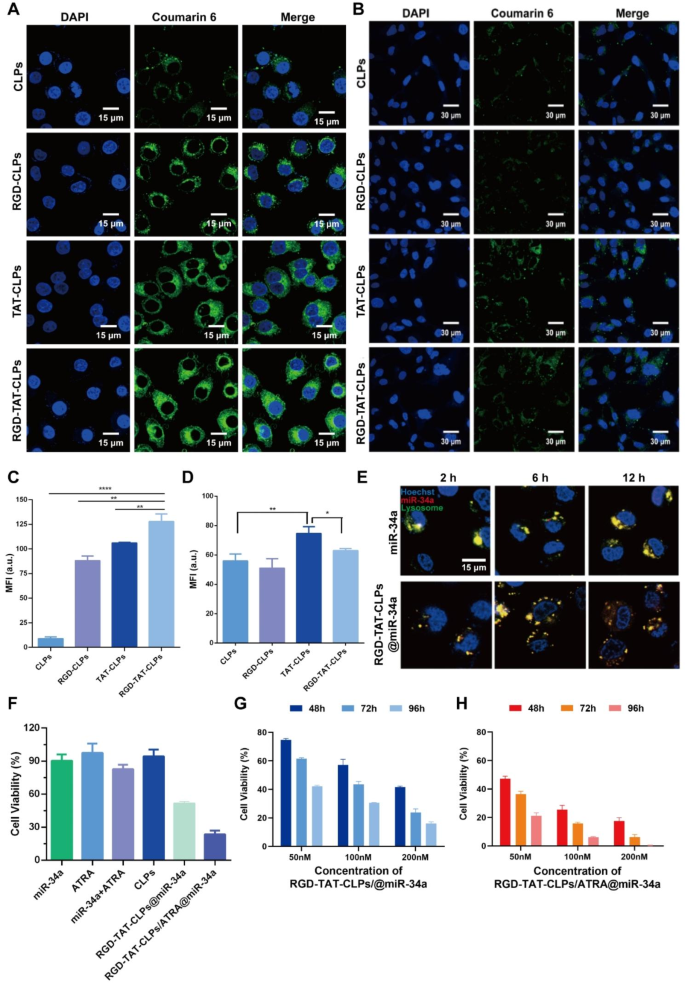
Mobile uptake and in vitro antitumor impact
After profitable preparation of the RGD-TAT-CLPs/ATRA@miR-34a, the mobile uptake and in vitro antitumor results have been evaluated utilizing human lung tumor cells A549. Outfitted with the dual-peptide modifications, the proposed nanosystem was anticipated to effectively goal tumor cells and be internalized. To display supply specificity, regular human bronchial epithelial cells (Beas-2b) have been employed for comparative evaluation. The liposomes have been fluorescently labeled with coumarin 6 to facilitate monitoring of their mobile distribution. As proven in Fig. 2A and 2C, in A549 cells, the intracellular inexperienced fluorescence in peptide-modified CLPs teams was a lot higher than CLPs teams, proving the upper CLPs uptake quantities induced by RGD and TAT modifications. As anticipated, the RGD-TAT-CLPs group demonstrated probably the most sturdy inexperienced fluorescence, exhibiting roughly 14.34 instances increased depth in comparison with the CLPs group. This statement highlights the synergistic results of RGD’s tumor cell concentrating on capability and TAT’s membrane permeation capacity. Nonetheless, it’s noteworthy that in Beas-2b cells, whereas the RGD modification didn’t considerably improve mobile uptake effectivity, a slight enchancment was noticed with the TAT modification when it comes to CLPs’ mobile uptake stage (Fig. 2B and 2D). These outcomes indicated that the RGD may particularly establish the tumor cells whereas the TAT may improve mobile internalization even in non-tumor cells. With this superior tumor cell concentrating on and uptake capability, extra ATRA and miR-34a have been anticipated to be intracellularly delivered, boosting the antitumor gene remedy.
Nonetheless, as one other intracellular supply problem of nanosystems, the lysosome seize and sequestration would possibly severely compromise their therapeutical effectivity [7]. Thus, the lysosome escape capacity of the proposed RGD-TAT-CLPs@miR-34a was evaluated. As proven in Fig. 2E, the colocalization of miR-34a and lysosomes have been imaged. In comparison with free miR-34a, the co-localization of RGD-TAT-CLPs@miR-34a and lysosome sequentially decreased as time glided by, indicating the lysosome escape capacity of RGD-TAT-CLPs. It might be defined by the proton-sponge impact of cationic DOTAP in CLPs. After being captured by lysosomes, the DOTAP might be protonated and induce the chloride ions inflow, which could result in the osmotic swelling and the bodily rupture of the lysosomal membrane.
Subsequently, the in vitro antitumor impact of the proposed RGD-TAT-CLPs/ATRA@miR-34a was decided. As proven in Fig. 2F, the free miR-34a couldn’t induce an apparent antitumor impact, which can be attributed to their instability within the cell tradition medium. The free ATRA may neither exhibit a cell-killing impact as a consequence of its low working focus. When the A549 cells have been handled with the mix of miR-34a and ATRA, a stronger cytotoxicity was proven, indicating that ATRA may improve the antitumor impact of miR-34a by augmenting the GJ capabilities to spice up the intercellular transport of miR-34a. Stunningly, when miR-34a was encapsulated within the RGD-TAT-CLPs, the cell cytotoxicity was considerably improved with cell viability reducing from about 90.36% to about 51.53%. This might be ascribed to the improved stability and mobile uptake of RGD-TAT-CLPs@miR-34a. Expectably, the strongest cell-killing impact was noticed within the RGD-TAT-CLPs/ATRA@miR-34a group, suggesting the gene remedy promotion impact of the GJ regulating technique.
Then, the concentration-dependent and time-dependent in vitro tumor progress inhibition impact of RGD-TAT-CLPs@miR-34a (Fig. 2G) and RGD-TAT-CLPs/ATRA@miR-34a (Fig. 2H) was additional investigated. As time went on, the cell viability in all of the teams clearly decreased, indicating the time-dependent method. In comparison with that of RGD-TAT-CLPs@miR-34a, the cell progress inhibition impact of RGD-TAT-CLPs/ATRA@miR-34a group was a lot higher with about solely 0.35% cell viability in 96 h below 200 nM, demonstrating the specified antitumor impact of RGD-TAT-CLPs/ATRA@miR-34 assisted by the GJ regulating technique. These outcomes collaboratively proved the improved antitumor impact by twin peptide-modified CLPs plus GJ perform regulation.
Mobile uptake and in vitro antitumor impact of RGD-TAT-CLPs/ATRA@miR-34a: (A-B) Mobile uptake of various formulations in A549 (A) and Beas-2b cells (B), the place inexperienced fluorescence represents completely different CLPs whereas blue fluorescence represents cell nucleus. (C-D) Semi-quantitative evaluation of A (C) and B (D). (E The CLSM co-localization photographs of RGD-TAT-CLPs@miR-34a and miR-34a with A549 cells lysosomes at completely different time factors. (F) Cell viability of A549 cells with completely different therapies. (G-H) In vitro A549 cell progress inhibition impact of RGD-TAT-CLPs@miR-34a (G) and RGD-TAT-CLPs/ATRA@miR-34a (H) with completely different miR-34a concentrations below completely different concentrations and time factors. (n = 6)
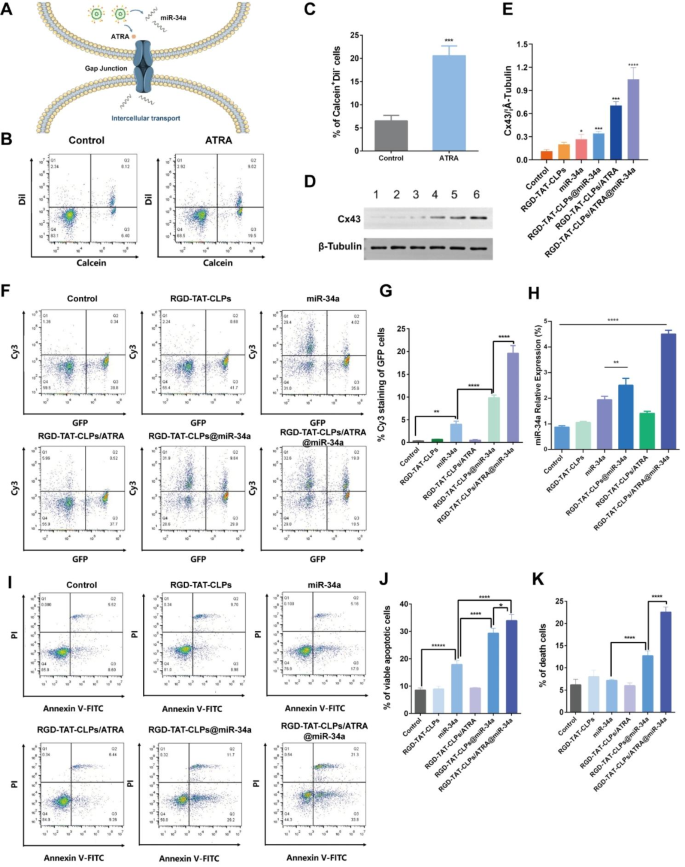
Gene remedy enhancement mechanism by GJ regulating technique
Based on the abovementioned outcomes, the proposed RGD-TAT-CLPs/ATRA@miR-34a exhibited a unprecedented antitumor impact within the A549 cell mannequin, which was attributed to the end result of the GJ regulating technique. When the RGD-TAT-CLPs/ATRA@miR-34a was intracellularly delivered, the GJ-relative Cx was anticipated to be upregulated by ATRA, which in flip enhanced the GJ-mediated miR-34a intercellular transport by the “by-stander impact”. The widespread miR-34a in tumor cells may induce cell apoptosis (Fig. 3A). To consolidate this speculation, a collection of experiments have been carried out.
Firstly, the GJ existence and the perform thereof have been evaluated by a circulation cytometry methodology, the place the “donor cells” stained with Calcein-AM and Dil-CM have been seeded into the clean “recipient cells”. Calcein-AM may solely be transported between donor and recipient cells by means of GJ whereas Dil-CM couldn’t be intercellularly transported. Thus, the share of the Calcein+Dil− cells might be employed to research the GJ capabilities. As proven in Fig. 3B and 3C, the ATRA remedy considerably elevated the Calcein+Dil− cells from about 6.4% to about 19.5%, indicating the enhancement of GJ in A549 cells. Then, the expression of Cx43, a vital GJ constituting Cx, was investigated with completely different therapies to additional validate the GJ-regulating impact of RGD-TAT-CLPs/ATRA@miR-34a (Fig. 3D and 3E). Surprisingly, the miR-34a and RGD-TAT-CLPs@miR-34a therapies barely upregulate the expression of Cx43, which may be ascribed to the difficult signaling mechanism. Apart from, the Cx43 expression in RGD-TAT-CLPs/ATRA and RGD-TAT-CLPs/ATRA@miR-34a group was a lot increased than different teams, indicating the payload may upregulate the Cx43 expression to boost the GJ perform. Subsequently, the impact of the improved GJ perform on miR-34a intercellular transport was decided by treating the “recipient cells” A549 transfected with a inexperienced fluorescent protein (GFP-A549) plus “donor cells” A549 cells handled with completely different Cy3-labelled miR-34a formulations (Fig. 3F and 3G). Thus, the intercellular miR-34a transport might be revealed by the share of Cy3+GFP+ cells. As proven, the free miR-34a remedy may barely enhance the Cy3+GFP+ cells proportion from about 0.34% to about 3.98%, indicating the weak GJ capabilities of A549 cells and the instability of free miR-34a. Nonetheless, the encapsulation of miR-34a into RGD-TAT-CLPs remarkably upregulated the miR-34a stage in GFP-A549 cells, which may be the results of miR-34a stability enhancement. Expectedly, the best Cy3+GFP+ cells proportion was exhibited within the RGD-TAT-CLPs/ARTA@miR-34a group, demonstrating the superior impact of GJ regulating technique on miR-34a intercellular transport. These findings collectively help the notion that miR-34a may be transported between cells by means of GJ, a course of additional enhanced by ATRA co-delivery.
Then, the relative miR-34a expression of the abovementioned A549-GFP recipient cells was investigated. As proven in Fig. 3H, just like the outcomes of miR-34a intercellular transport evaluation, the improved miR-34a expression ranges have been noticed in RGD-TAT-CLPs@miR-34a and RGD-TAT-CLPs/ATRA@miR-34a teams, and the best expression stage was proven within the latter. These outcomes additional validated the feasibility of dual-peptide modified CLPs and the GJ regulating technique within the enhanced supply of miR-34a. Afterward, the consequences of miR-34a expression on cell apoptosis have been studied. It was reported that the p53-related cell apoptosis signaling pathway may be triggered by the miR-34a expression. The Annexin V-FITC/PI circulation cytometry methodology was employed to research the share of viable apoptotic cells (Annexin V-FITC+PI− cells) and loss of life cells (Annexin V-FITC+PI+ cells). As proven in Fig. 3I-Okay, miR-34a remedy enhanced the viable apoptotic cell proportion from 8.6 to 17.9%, indicating its apoptosis-inducing perform. Nonetheless, a lot vital cell apoptosis (about 29.2%) and loss of life (about 11.7%) have been induced by the RGD-TAT-CLPs@miR-34a, demonstrating the improved stability and supply of miR-34a. The best cell apoptosis and cell loss of life have been brought on by the RGD-TAT-CLPs/ATRA@miR-34a group, suggesting the boosted cell-killing effectivity induced by GJ regulation mediated miR-34a expression. Taken collectively, the miR-34a-induced gene remedy was validated to be boosted by the ATRA inflicting GJ regulation and dual-peptide modified CLPs supply.
Moreover, in accordance with the hole junction regulatory technique, it’s noteworthy {that a} strategically designed sequential launch system may probably exhibit a profound antitumor impact, whereby the administration of ARTA would precede that of miR-34a. On this situation, the preliminary launch of ARTA would successfully upregulate Cx43 expression to facilitate hole junction opening, subsequently enabling the next launch of miR-34a to traverse by means of tumor cells and induce apoptosis. This revolutionary design might be topic to forthcoming investigation.
Gene remedy enhancement mechanism by GJ regulation technique: (A) Schematical illustration of the mechanism of GJ regulation enhanced miR-34a transport. (B-C) The circulation cytometry outcomes of donor and recipient A549 cells labeled with Calcein-AM and Dil-CM staining (B) and its semi-quantitative evaluation of Calcein+Dil− cells (C). (D-E) The Western blot outcomes of Cx43 in A549 cell after completely different therapies (D) and its semi-quantitative evaluation (E), (1) Management, (2) RGD-TAT-CLPs, (3) miR-34a, (4) RGD-TAT-CLPs@miR-34a, (5) RGD-TAT-CLPs/ATRA, (6) RGD-TAT-CLPs/ATRA@miR-34a. (F-G) The intercellular transport of miR-34a in A549 cells analyzed with circulation cytometry (F) and its semi-quantitative evaluation of GFP+Cy3+ cells (G). (H) The miR-34a expression stage in A549 cells with completely different therapies. (I-Okay) The Annexin V-FITC/PI circulation cytometry outcomes of A549 cells handled with completely different therapies (I) and its semi-quantitative outcomes (J and Okay) (n = 3)
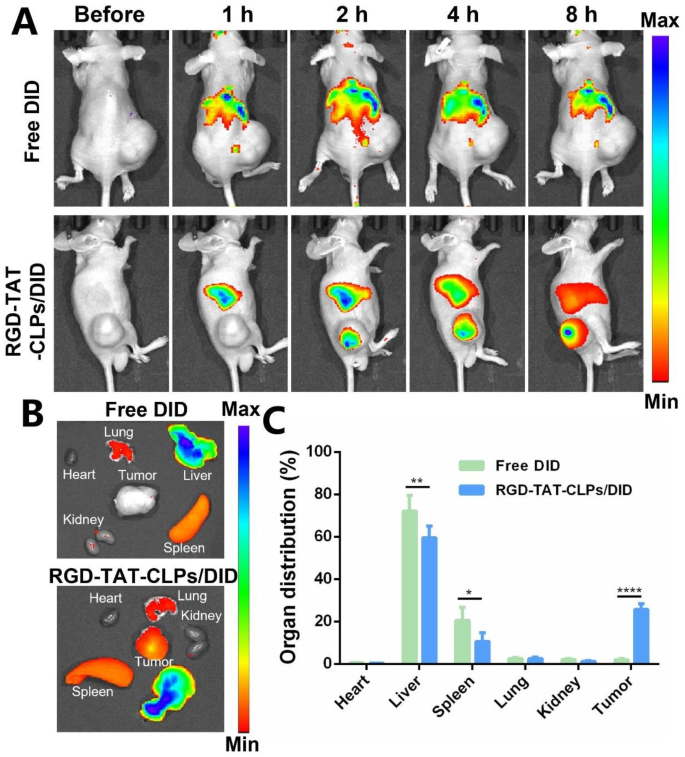
In vivo anti-tumor impact and mechanism on transplant subcutaneous Tumor mannequin
The specified in vitro anti-tumor impact of RGD-TAT-CLPs/ATRA@miR-34a impressed us to judge its in vivo antitumor impact, which was first validated on a transplant subcutaneous A549 tumor mannequin. Earlier than evaluating the tumor progress inhibition effectivity, the in vivo tumor-targeting capacity of dual-peptide modifications was investigated. The fluorescence probe DID was labeled on the RGD-TAT-CLPs to hint their in vivo accumulation, and the free DID group was set as a comparative group (Fig. 4). After intravenous injection, the RGD-TAT-CLPs/DID shortly collected within the tumor web site inside 2 h whereas the tumor fluorescence within the free DID group was not apparent (Fig. 4A). The excessive tumor accumulation in RGD-TAT-CLPs/DID group was maintained till 8 h, indicating its superior tumor concentrating on capability. An analogous conclusion might be drawn from the ex vivo outcomes, the place the best DID accumulation was recorded in tumors apart from the liver in RGD-TAT-CLPs/DID teams whereas no distinct tumor concentrating on capacity was discovered within the free DID group (Fig. 4B and 4C). As well as, the CLPs/DID group was additional added to judge the perform of dual-peptide modification (Fig. S11). A considerably enhanced tumor drug accumulation was additionally exhibited within the RGD-TAT-CLPs/DID group in comparison with the CLPs/DID group. These outcomes might be defined by the tumor cell concentrating on capability of RGD and the mobile transmembrane capability of TAT. Taken collectively, RGD-TAT-CLPs may function an efficient service to ship medicine to tumor lesions, facilitating the antitumor impact.
In vivo concentrating on capacity analysis of RGD-TAT-CLPs: (A) The fluorescence photographs of subcutaneous tumor-bearing mice after the intravenous injection of free DID or RGD-TAT-CLPs/DID at completely different instances. (B-C) The fluorescence picture (B) and its semi-quantitative evaluation outcomes (C) of main organs and tumors derived from tumor-bearing mice at 8 h. (n = 3)
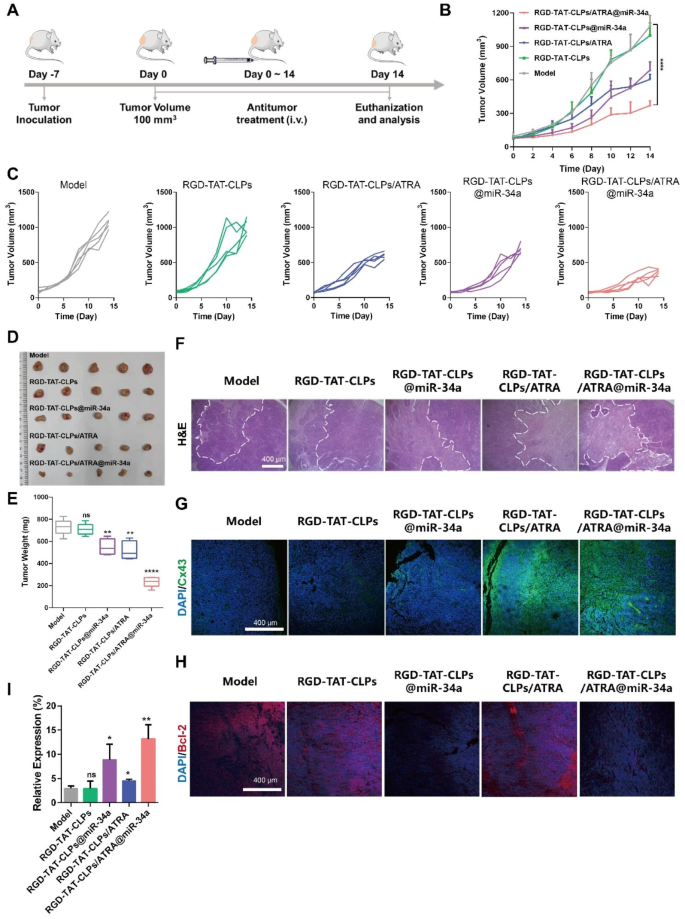
Then, the in vivo antitumor results have been evaluated (Fig. 5A). There have been 5 teams: Mannequin, RGD-TAT-CLPs, RGD-TAT-CLPs/ARTA, RGD-TAT-CLPs@miR-34a and RGD-TAT-CLPs/ATRA@miR-34a. The tumor quantity curves are proven in Fig. 5B and 5C. As proven, the tumor within the mannequin group was enlarged in an exponential method with the tumor quantity exceeding 1000 mm3 on the 14th day. No apparent inhibition impact was exhibited within the RGD-TAT-CLPs group, indicating the negligible antitumor impact of the nanocarrier. Nonetheless, tumor progress was considerably inhibited within the different three teams. The loading of ATRA or miR-34a into RGD-TAT-CLPs may to some extent exert some antitumor impact. The tumor inhibition impact of ATRA may be ascribed to its tumor plasticity-regulating capacity whereas that of miR-34a was the results of its tumor apoptosis-inducing capacity. Restricted by the poor tumor cells penetration of miR-34a, the tumor inhibition impact of RGD-TAT-CLPs@miR-34a was not happy. Compared, the strongest tumor-killing impact was proven within the RGD-TAT-CLPs/ARTA@miR-34a group, with the slowest enhance in tumor quantity in all mice and a mean tumor quantity of about 370 mm3 after 14 days, which may be attributed to the improved miR-34a supply into deep tumors by the GJ regulating technique. These outcomes have been confirmed by the tumor inhibition fee outcomes (Fig. S12). To additional validate the conclusion, the tumors have been excised on the 14th day, which have been then imaged and weighed. As proven in Fig. 5D and 5E, the bottom common tumor weight (about 235.00 mg) was recorded within the RGD-TATPCLPs/ARTA@miR-34a group, which was a lot decrease than that within the mannequin group (about 729.40 mg), suggesting a 2.78-time increased therapeutical effectivity than RGD-TAT-CLPs@miR-34a group. From the abovementioned outcomes, the effectiveness of the GJ regulating technique on gene remedy enhancement in vivo was strongly supported.
Afterward, the excised tumors have been sliced for pathological examination to research their antitumor mechanism. Based on the outcomes of H&E staining, the best tumor broken space and the bottom tumor cells density have been proven within the RGD-TAT-CLPs/ARTA@miR-34a group, indicating its strongest tumor cell-killing impact (Fig. 5F). To validate the supply of GJ regulating technique mediated miR-34a to deep tumor tissues, the Cx43 staining was employed to detect the GJ perform in tumor tissues (Fig. 5G). As anticipated, the mannequin group with low Cx43 expression exhibited impaired GJ perform. Nonetheless, remedy with RGD-TAT-CLPs/ARTA and RGD-TAT-CLPs/ARTA@miR34a considerably enhanced inexperienced fluorescence depth, indicating upregulated Cx43 expression in tumor tissues. This statement additional helps the notion of improved GJ capabilities. The augmented GJ perform is prone to facilitate intercellular transport of miR-34a and subsequently induce tumor apoptosis. As revealed within the staining photographs of Bcl-2, a miR-34a-related apoptosis-inhibition protein, the apoptosis fee was remarkably enhanced within the RGD-TAT-CLPs@miR-34a and RGD-TAT-CLPs/ATRA@miR-34a teams, indicating the apoptosis-inducing impact of miR-34a (Fig. 5H). Lastly, the miR-34a expression stage in tumor tissues was quantitatively measured by qRT-PCR (Fig. 5I). According to the in vitro outcomes, the best miR-34a expression stage was revealed within the RGD-TAT-CLPs/ARTA@miR-34a group, which was about 4.49 instances increased than the mannequin group. The miR-34a expression was additionally increased than the RGD-TAT-CLPs@miR-34a group, suggesting the robustness of the GJ regulating technique. These outcomes collaboratively proved the nice antitumor results of the proposed RGD-TAT-CLPs/ARTA@miR-34a.
In vivo anti-tumor impact of RGD-TAT-CLPs/ARTA@miR-34a: (A) Schematical illustration of in vivo antitumor impact analysis in transplant subcutaneous A549 tumor mannequin. (B-C) A549 tumor quantity curves of mice in 14 days with completely different therapies. (D-E) Tumors photographs (D) and common weights (E) of tumors dissected from tumor-bearing mice on the 14th day after completely different therapies. (F-H) H&E staining (F), Cx43 staining (G) and Bcl-2 (H) staining photographs of tumors from mice with completely different therapies. (I) The relative expression of miR-34a in tumors after completely different therapies on the 14th day. (n = 5)
The security of the proposed RGD-TAT-CLPs/ARTA@miR-34a was additionally evaluated in the course of the therapeutical interval. No apparent physique weight change was recorded (Fig. S13). Based on the organ weight effectivity evaluation, the primary organs together with coronary heart, liver, spleen, lung and kidney weren’t clearly broken, which is also discovered within the look (Figs. S14 and S15). Then, the primary organs have been excised for H&E evaluation. As proven in Fig. S16, no noticeable abnormalities, corresponding to fibrosis, infiltration, or irritation, have been discovered within the main organs of handled mice, indicating the nice security of the nanosystem. Lastly, revealed by the hemogram examination, no vital influence of the proposed RGD-TAT-CLPs/ARTA@miR-34a on blood cells and capabilities was discovered (Fig. S17). Taken collectively, the offered RGD-TAT-CLPs/ARTA@miR-34a didn’t trigger any observable systematical toxicity.
Building and characterization of RGD-TAT-CLPs/ARTA@miR-34a-DPIs
Given its fascinating lung accumulation capacity and low enzyme exercise within the respiratory system, pulmonary supply of gene therapeutic brokers holds promise for exerting a stronger antitumor impact in comparison with different supply routes for lung tumor remedy. In gentle of their solid-state kind that enhances drug loading and maintains gene stability, DPIs have been acknowledged as an excellent pulmonary supply system. Subsequently, we aimed to develop DPI formulations of the synthesized RGD-TAT-CLPs/ARTA@miR-34a complicated. For this objective, lactose (Lac), an FDA-approved DPI service materials, together with hydroxypropyl-β-cyclodextrin (HP-β-CD), a usually acknowledged as secure (GRAS) materials by FDA, have been chosen as service supplies for RGD-TAT-CLPs/ARTA@miR-34a-DPIs.
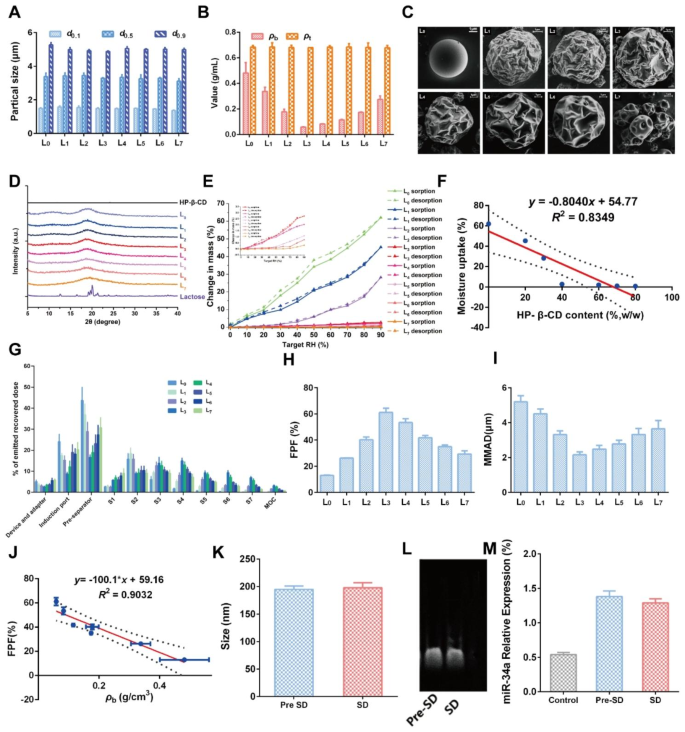
To acquire desired DPIs with happy aerosolization properties, a collection of Lac- HP-β-CD ratios was set to arrange formulations L0-L7 by a twig drying (SD) methodology (Desk 1). Then, the fundamental physicochemical properties of the ready DPIs have been investigated to find out the optimized formulation. As proven in Fig. 6A, the particle dimension of all of the formulations was related, with a d0.1 of about 1.5 μm and a d0.9 of about 5 μm, suggesting the nice potential for supply to the decrease airway. It was thought of that the suitable particle dimension for inhalation needs to be between 0.5 and 5 μm, which might assure particles deposition within the deep lung after a number of deposition mechanisms like collision with the airway floor, gravitational sedimentation and diffusional deposition [39]. Then, the majority density (ρb) and faucet density (ρt) have been measured (Fig. 6B). No apparent distinction in ρt was noticed whereas ρb decreased from L0 to L3 however elevated from L4 to L7, indicating the addition of HP-β-CD would possibly exert vital results. Amongst them, the smallest ρb was recorded in L3, implying the perfect dispersibility and flowability. Additional, the morphology of DPIs particles was noticed by scanning an digital microscope (SEM), and the outcomes are proven in Fig. 6C. An ideal spherical and clean particle was noticed in L0 with out HP-β-CD addition. With the addition of HP-β-CD, the particle floor turned rougher and corrugated in L1-L6, which was extra just like the date stone-like morphology of the HP-β-CD service (L7). Nonetheless, the floor morphology of L4-L7 was too corrugated to take care of the spherical form, which could induce the particles to cross-link to one another, leading to worse pulmonary supply efficiency. Then, the crystallinity of DPIs was analyzed by Powder X-Ray Diffraction (PXRD). As proven in Fig. 6D, a typical α-crystallinity was present in Lac whereas HP-β-CD exhibited an amorphous state. For DPIs, no apparent diffraction peaks have been present in all of the formulations, indicating that the DPIs particles have been within the amorphous state. Apart from, the hygroscopicity of DPIs was decided as water adsorption would possibly considerably influence the DPIs particles stability, in flip influencing their aerosolization efficiency. The burden acquire of L0 was about 50.7% at 90% RH, suggesting the nice hygroscopicity of Lac service (Fig. 6E). Nonetheless, with the addition of HP-β-CD, the water adsorption was remarkably inhibited with lower than 5% in L1-L7, which may be attributed to the moisture-resistance nature of HP-β-CD. It was revealed that the hygroscopicity was negatively correlated with the HP-β-CD addition quantity (Fig. 6F). The above outcomes collaboratively demonstrated that the right addition of the HP-β-CD may improve the potential pulmonary supply efficiency of DPIs by growing the floor roughness and inhibiting water adsorption, whereas L1-L3 might possess the perfect efficiency.
Then, the in vitro aerosolization efficiency of RGD-TAT-CLPs/ARTA@miR-34a-DPIs was investigated by the following era impactor (NGI), and the outcomes are proven in Fig. 6G. The small geometric customary deviation (GSD) values of all formulations recommended a slender particle dimension distribution (Fig. S18). In comparison with different formulations, a a lot increased decrease airway deposition (S3 – S7) and a decreased deposition within the machine and adaptor, induction port, pre-separator and S1 – S2 have been revealed in L3. Additional, the best (about 61.17%) effective particle fraction (FPF) and applicable (about 2.16 μm) mass median aerodynamic diameter (MMAD) was additionally validated in L3. Herein, FPF was outlined because the fraction of drug successfully deposited within the lung, whereas MMAD was considered the aerodynamic dimension of particles with a mass cumulative proportion of fifty% (Fig. 6H and I) [40]. These outcomes strongly supported that the optimized pulmonary supply efficiency can be achieved by L3. And a unfavorable correlation between the FPF worth and the ρb of DPIs was additionally revealed (R2 = 0.9032), which was per different experiences (Fig. 6J) [40].
Having constructed the optimized RGD-TAT-CLPs/ARTA@miR-34a-DPIs, the drug loading capability and stability have been investigated earlier than and after the SD course of. The scale and floor Zeta Potential of loaded RGD-TAT-CLPs/ARTA@miR-34a have been decided earlier than and after SD (Fig. 6Okay and Fig. S19). It was revealed that no apparent change in dimension and Zeta Potential was noticed earlier than and after SD, indicating the nice recoverability of RGD-TAT-CLPs/ARTA@miR-34a when delivered into the lung. Then, the steadiness of the encapsulated medicine was analyzed earlier than and after the SD course of. As proven in Fig. 6L, the exercise of loaded miR-34a was not impacted by the SD course of with related electrophoresis photographs. Additional, the qRT-PCR outcomes revealed that the relative expression stage of miR-34a was not influenced by the SD course of, which was nonetheless a lot increased than the management group (Fig. 6M). As well as, the ATRA loading and its launch profile have been additionally maintained after the DPIs building course of (Fig. S20). Taken collectively, a negligible influence of the SD course of on the steadiness and drug loading of RGD-TAT-CLPs/ARTA@miR-34a was discovered, indicating its excessive feasibility to function a pulmonary supply platform for antitumor gene remedy.
Based mostly on what we mentioned above, the proposed RGD-TAT-CLPs-DPIs have been geared up with nice pulmonary supply efficiency and desired gene supply stability. Thus, it was believed that the offered DPIs system may function a pulmonary gene supply platform. For additional in vitro and in vivo research, extra gene remedy can be supported by the proposed DPIs platform for the remedy of different lung native illnesses.
Building and characterization of RGD-TAT-CLPs/ARTA@miR-34a-DPIs: (A-E) Particle dimension measurements (A), bulk density and faucet density (B), floor morphology photographs (C), PXRD patterns (D) and water adsorption curves (E) of RGD-TAT-CLPs/ARTA@miR-34a-DPIs of various formulations. (F) The correlation between moisture uptake and the HP-β-CD content material in RGD-TAT-CLPs/ARTA@miR-34a-DPIs. (G) The in vitro deposition distribution of RGD-TAT-CLPs/ARTA@miR-34a-DPIs of various formulations. (H-I) The FPF (H) and MMAD (I) of various formulations. (J) The correlation between FPF and ρb worth of DPIs. (Okay) The particle dimension of RGD-TAT-CLPs/ARTA@miR-34a earlier than and after SD. (L-M) The gel electrophoresis photographs (L) and qRT-PCR detection outcomes (M) of RGD-TAT-CLPs/ATRA@miR-34a earlier than and after SD