Pyroptosis occures in microglia and Treg cell is elevated after spinal wire damage
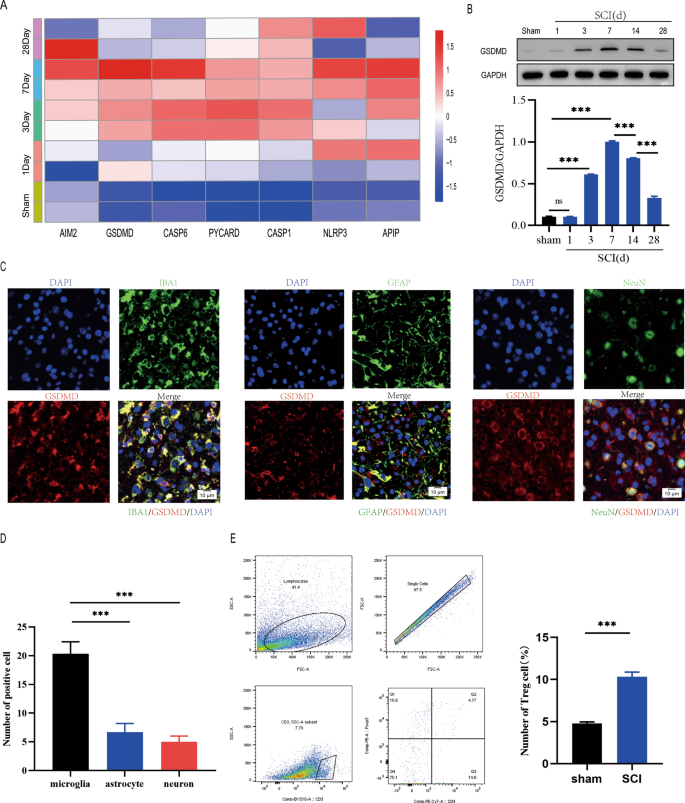
To research the spatiotemporal traits of pyroptosis after spinal wire damage, we carried out a severe of experiments. Bioinformatics evaluation of GSE5296 revealed the expression of pyroptosis-related genes elevated in mice with spinal wire damage than in sham-operated mice on day 1 and was highest on day 7, suggesting that pyroptosis peaked on day 7 after SCI(Fig. 1A). GSDMD protein ranges have been additionally markly elevated on day 7(Fig. 1B). For the reason that spinal wire incorporates a wide range of cell sorts, together with astrocytes (GFAP+), microglia (IBA1+), and neurons (NeuN+), we investigated whether or not pyroptosis happens in a number of sorts of cells after spinal wire damage. Immunofluorescence evaluation revealed that pyroptosis primarily occured in microglia within the injured spinal wire and was lessly occured in different cell sorts(Fig. 1C, D). Moreover,We ascertained a increas inhabitants of the Foxp3 + Treg cell within the injured spinal wire utilizing circulate cytometry at days 7 after spinal wire damage(Fig. 1E). These outcomes recommend that Treg cell could also be necessary within the regulation of microglia pyroptosis following spinal wire damage.
Pyroptosis happens in microglia following spinal wire damage. A pyroptosis-related gene expression patterns at days 1, 3, 7, and 28 after SCI; B Consultant immunoblots displaying the extent of GSDMD protein at Days 1, 3, 7, 14, and 28 following SCI. measurement of the relative GSDMD protein ranges (n = 3); C Immunofluorescence of the spinal wire 7 days after damage reveals that GSDMD is usually expressed in microglia (IBA1) however much less in neurons (NeuN) or astrocytes (GFAP); D Quantification of fluorescence depth of GSDMD at Day 7 postinjury(n = 3); E Stream cytometry demonstrating that Treg cell infiltration is considerably elevated within the spinal wire 7 days after damage(n = 3)
Particular Treg-cell ablation in Foxp3DTR mice promotes microglia pyroptosis in vivo
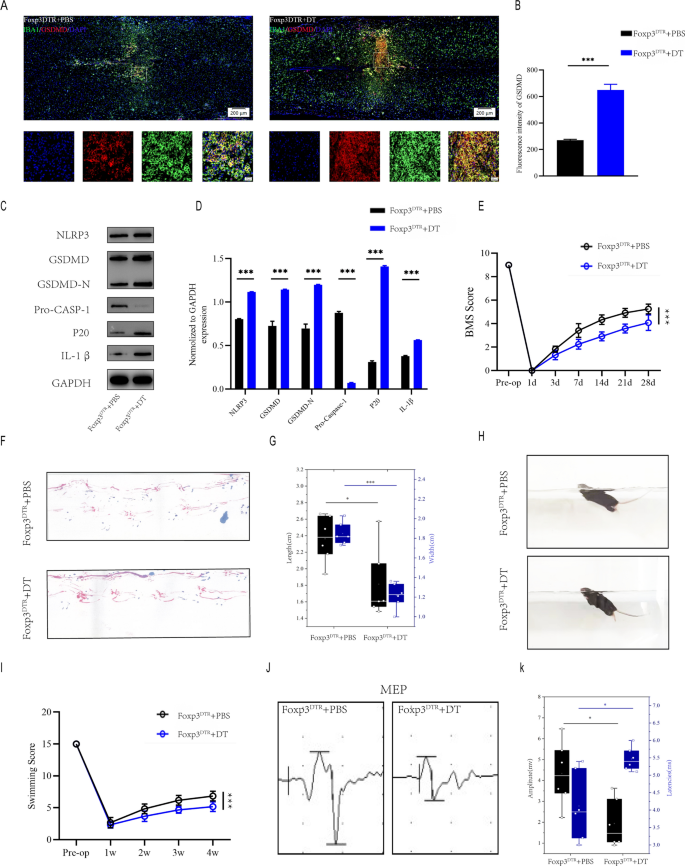
To evaluate the impact of Treg cells on microglial pyroptosis after spinal wire damage, we selectively depleted Treg cells by diphtheria toxin (DT) injections in Foxp3DTR transgenic mice that specific the DT receptor underneath management of the Foxp3 promoter. Immunofluorescence of damage areas revealed that Foxp3DTR + DT mice had extra IBA1+/GSDMD+ microglia than Foxp3DTR + PBS mice(Fig. 2A, B). Furthermore, The severity of pyroptosis was additionally decided by utilizing westernblot to detect NLRP3, GSDMD, GSDMD-N, Professional-CASP-1,caspase1(p20) and IL-1β ranges within the damage middle 7 days after spinal wire damage(Fig. 2C, D). Foxp3DTR + DT mice have considerably extra in depth microglia pyroptosis than Foxp3DTR + PBS mice one week after SCI, implying that Treg cell knockout promote microglial pyroptosis in vivo. BMS rating demonstrated that Foxp3DTR + DT mice recovered considerably lower than Foxp3DTR + PBS mice through the 4-week post-SCI restoration course of (Fig. 2E). Foxp3DTR + DT mice had much less movementr coordination and fewer efficient gait restoration, as proven by footprint evaluation, supporting the findings of the BMS (Fig. 2F, G). Foxp3DTR + DT mice additionally had poorer purposeful restoration in Swimming take a look at (Fig. 2H, I).Moreover, electrophysiological evaluation revealed that Foxp3DTR + DT mice had smaller amplitudes and longer latencies of motor evoked potentials (MEPs) after SCI than Foxp3DTR + PBS mice(Fig. 2J, Okay). These behavioral checks point out that deletion of Treg cell in mice following SCI leads to poor purposeful restoration. These findings recommend that Treg cell deficiency causes in depth microglial pyroptosis and impairs purposeful restoration following spinal wire damage.
Knokout of Treg cell results in widespread microglia pyroptosis and impairs purposeful restoration after SCI. A Consultant immunofluorescence photos for GSDMD(pyroptosis) and IBA1(microglia) expression in Foxp3DTR + PBS and Foxp3DTR + DT mice at Day 7 postinjury; B Quantification of fluorescence depth at Day 7 postinjury(n = 3); C Consultant immunoblots exhibiting ranges of pyroptosis-related protein in injured spinal wire at days 7 postinjury in Foxp3DTR + PBS and Foxp3DTR + DT mice; D Quantification of relative ranges of pyroptosis-related protein (n = 3); E BMS scores throughout 28 days of restoration after SCI reveal impaired purposeful restoration in Foxp3DTR + DT mice (n = 6); F Footprint evaluation at Day 28 postinjury reveal impaired purposeful restoration in foxp3DTR + DT mice; G The footprints quantifcation of mice strolling 28 days after SCI(n = 6); H Swimming take a look at at Day 28 postinjury reveal impaired purposeful restoration in foxp3DTR + DT mice; I Louisville Swim Scale in Foxp3DTR + PBS and Foxp3DTR + DT mice at Day 28 postinjury(n = 6); J MEP evaluation was used as electrophysiological evaluation in Foxp3DTR + PBS and Foxp3DTR + DT mice at Day 28 postinjury; Okay Quantification of peak-to-peak MEP amplitudes and latencies in Foxp3DTR + PBS and Foxp3DTR + DT mice (n = 6)
Elevated Treg-cell infiltration in spinal wire attenuates microglia pyroptosis in vivo
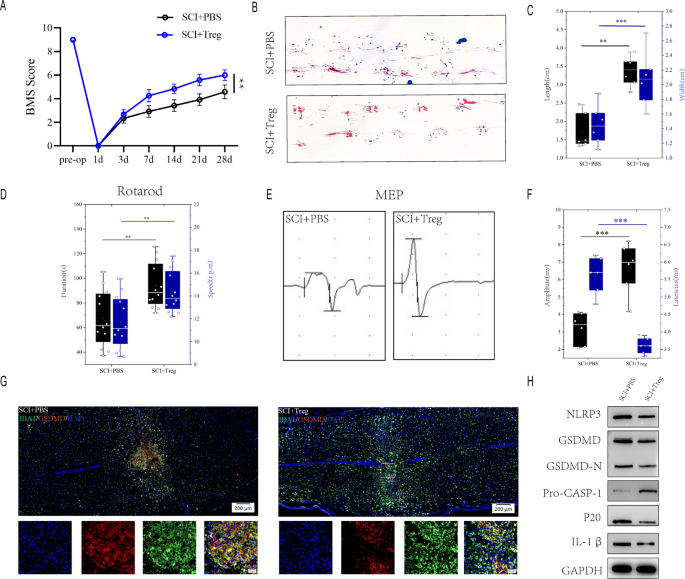
To additional assess the impact of Treg cells on microglial pyroptosis after spinal wire damage, We injected Treg cells within the tail vein of mices instantly after spinal wire damage, and the infiltration of Foxp3 + cells within the spinal wire was considerably elevated on day 7 after spinal wire damage (Determine S1A).BMS rating demonstrated that SCI + PBS mice recovered considerably lower than SCI + Treg mice through the 4-week post-SCI restoration course of (Fig. 3A). SCI + PBS mice had much less movementr coordination and fewer efficient gait restoration, as proven by footprint evaluation, supporting the findings of the BMS ((Fig. 3B, C). Rotarod testing for posterior limb and trunk equilibrium exhibited superior motor restoration in SCI + Treg mice (Fig. 3D). Electrophysiologic take a look at additionally revealed that SCI + PBS mice had smaller amplitudes and longer latencies of motor evoked potentials (MEPs) after SCI than SCI + Treg mice (Fig. 3E, F). These behavioral checks point out that elevated infiltration of Treg cell in mice following SCI leads to higher purposeful restoration. SCI + Treg mice have considerably much less microglia pyroptosis than SCI + PBS mice one week after SCI (Fig. 3G). A quantitative evaluation of IBA1+/GSDMD+ areas on the damage web site revealed that SCI + Treg mice had much less IBA1+/GSDMD+ microglia than SCI + PBS mice (Extra file 1: Determine S1B). Furthermore, The severity of pyroptosis was additionally decided by utilizing westernblot to detect NLRP3, GSDMD, GSDMD-N, Professional-CASP-1,p20 and IL-1β ranges within the damage middle 7 days after spinal wire damage(Fig. 3H). SCI + Treg mice had considerably decrease ranges of NLRP3, GSDMD, GSDMD-N, caspase1(p20) and IL-1β expression than SCI + PBS mice (Extra file 1: Determine S1C). These findings recommend that elevated Treg cell promotes purposeful restoration following spinal wire damage and attenuates microglial pyroptosis.
Elevated-infiltration of Treg cell in spinal wire promotes purposeful restoration and limits microglia pyroptosis after SCI. A BMS scores throughout 28 days of restoration after SCI reveal higher purposeful restoration in SCI + Treg mice (n = 6); B Footprint evaluation at Day 28 postinjury reveal higher purposeful restoration in SCI + Treg mice(n = 6); C The footprints quantifcation of mice strolling 28 days after SCI; D Rotarod testsat Day 28 postinjury reveal higher purposeful restoration in SCI + Treg mice(n = 12); E MEP evaluation was used as electrophysiological evaluation in SCI + PBS and SCI + Treg mice at Day 28 postinjury (n = 6); F Quantification of peak-to-peak MEP amplitudes and latencies in SCI + PBS and SCI + Treg mice. G Consultant immunofluorescence photos for GSDMD (pyroptosis) and IBA1 (microglia) expression in SCI + PBS and SCI + Treg mice at Day 7 postinjury. H Consultant immunoblots displaying ranges of pyroptosis-related protein in injured spinal wire at days 7 postinjury in SCI + PBS and SCI + Treg mice
Treg cell-derived exosomes attenuates pyroptosis in BV2 microglia in vitro
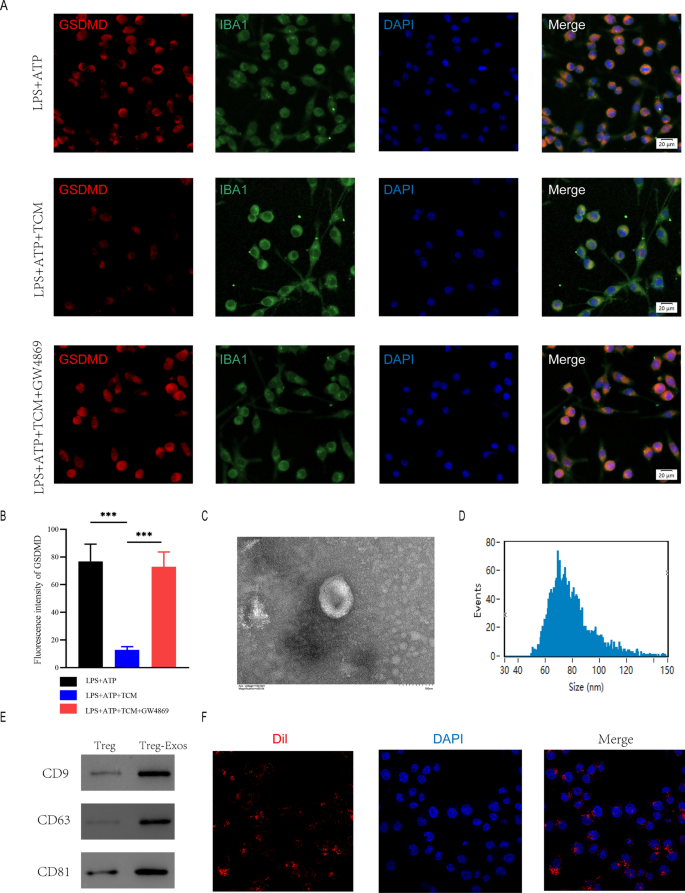
To additional examine the mechanism of microglia pyroptosis inhibition by Treg cells, we collected Treg-cell conditioned medium(TCM) after Treg cells have been stimulated with anti-CD3 (1ug/ml) and anti-CD28 (10ug/ml). BV2 cells have been co-cultured with TCM earlier than being handled with LPS + ATP. microglia have been immunofluorescently stained for IBA1 and GSDMD to evaluate pyroptosis. GSDMD expression was significantly decrease within the LPS + ATP + TCM group compared to the group that obtained solely LPS + ATP remedy. As a result of exosomes contribute considerably in intercellular communication by transferring hereditary materials, we speculated Treg cells attenuates pyroptosis in microglia by secreting exosomes. So as to forestall Treg cells from secreting exosomes, we used GW4869. Co-cultured TCM pre-treated GW4869 restored the expression ranges of GSDMD (Fig. 4A, B).
Treg-derived exosomes suppressed microglia pyroptosis. A Consultant immunostaining picture of IBA1 and GSDMD within the microglia; B Quantification of fluorescence depth(n = 3); C Morphology of exosomes underneath TEM (50–150 nm); D NTA evaluation exhibit exosomes measurement; E Western blot evaluation of exosome floor markers; F The pink fuorescence dye Dil-labeled exosomes was uptaken into microglia
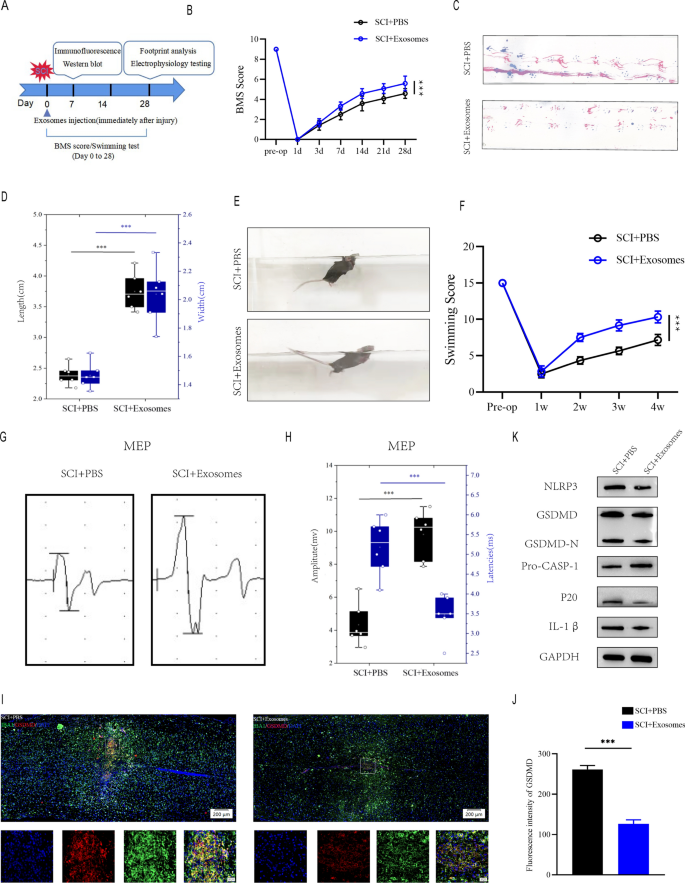
Exosomes derived from Treg cells help within the recovering of motor efficiency and attenuates pyroptosis following SCI
To additional discover the perform of Treg cell-derived exosomes within the spinal wire damage micro-environment and the underlying impact on microglia pyroptosis, we extracted exosomes (Treg-Exos) from Treg cells tradition supernatant and recognized them utilizing transmission electron microscopy (TEM), nanoparticle monitoring evaluation (NTA) and western blot. TEM confirmed basic nanoparticles with diameters between 50 and 150 nm, and NTA confirmed a semblable measurement distribution (Fig. 4C, D). Exosome floor markers like CD9, CD63, and CD81 have been detected by Western blot (Fig. 4E). Exosomes that have been Dil-labeled have been taken up by microglia (Fig. 4F). The above investigations verified the exosome seperated from Treg supernatants.
Exosomes have been injected without delay following SCI, and behavioral checks have been performed on the occasions specified (Fig. 5A). In accordance with BMS behavioral evaluation, Treg-Exos injection improved motor perform scores within the hind limbs following spinal wire damage (Fig. 5B). Footprint evaluation, swimming checks and MEPs all yielded related outcomes, with Treg-Exos injection leading to longer strides, larger MEP amplitude, smaller physique and water floor angles, and a extra upturned tail(Fig. 5C–H). When Treg-exos-treated group have been in comparison with PBS group, immunofluorescence staining revealed a lower in IBA-1 + /GSDMD + cells and fluorescence depth of GSDMD (Fig. 5I, J).Furthermore, Treg-exos-treated group had considerably decrease ranges of NLRP3, GSDMD, GSDMD-N,p20 and IL-1β expression than PBS group(Fig. 5Okay and Extra file 1: Determine S2A).
Exosomes derived from Treg cell promote motor perform restoration and supress microglia pyroptosis after SCI in vivo. A The experimental scheme for exosomes injection after SCI; B BMS was used to functionally grade mice in numerous teams as much as 28 days post-injury. C Footprint evaluation at Day 28 postinjury reveal higher purposeful restoration in SCI + Exosomes mice; D The footprints quantifcation of mice strolling 28 days after SCI(n = 6); E Swimming take a look at at Day 28 postinjury reveal higher purposeful restoration in SCI + Exosomes mice; F Louisville Swim Scale rating in SCI + PBS and SCI + Exosomes mice at Day 28 postinjury(n = 6); GMEP evaluation was used as electrophysiological evaluation in SCI + PBS and SCI + Exosomes mice at Day 28 postinjury; H Quantification of peak-to-peak MEP amplitudes and latencies in SCI + PBS and SCI + Exosomes mice (n = 6); I Consultant immunofluorescence photos for GSDMD(pyroptosis) and IBA1(microglia) expression in SCI + PBS and SCI + Exosomes mice at Day 7 postinjury; J Quantification of fluorescence depth at Day 7 postinjury (n = 3); Okay Consultant immunoblots displaying ranges of pyroptosis-related protein in injured spinal wire at days 7 postinjury in SCI + PBS and SCI + Exosomes mice
Furthermore, The outcomes of immunofluorescence confirmed that Treg-exosomes had higher therapeutic efficacy when it comes to treating microglia pyroptosis examine with Treg cell remedy, which can be as a result of traits of smaller particle measurement and better membrane permeability of exosomes, which allowed them to simply cross the blood-spinal barrier, thus exerting a greater impact on inhibiting microglia pyroptosis (Extra file 1: Determine S2B-C).
Taken collectively, these findings recommend that Treg-Exos can promotes purposeful restoration after SCI and reduce the onset of microglial pyroptosis.
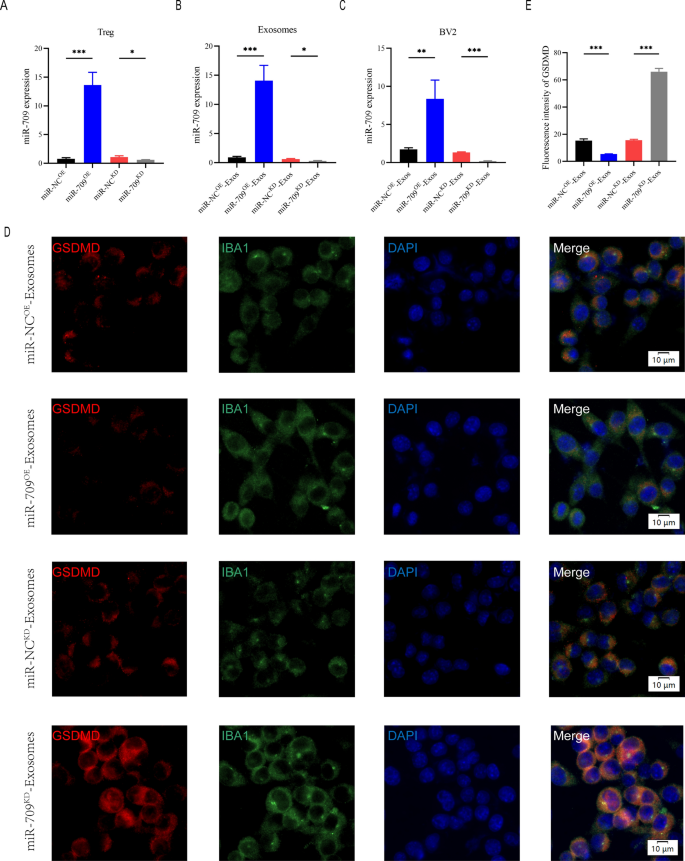
Exosomes ship miR-709 to microglia
Treg-Exos promotes purposeful restoration after SCI and inhibits the onset of microglial pyroptosis, in response to in vivo and in vitro research. Exosomal miRNAs have been demonstrated in prior analysis to have regulating actions on course cells and could also be essential for the alter of organic processes. miR-709 was screened by taking the intersection of the highest 5 expressed miRNAs in Treg cells and Treg-Exos (Determine S3A). Furthermore, miR-709 was markly elevated in Treg-Exos-treated BV2 cells, indicating that exosomes can switch miR-709 from Treg cells to BV2 cells (Extra file 1: Determine S3B).
Exosomes suppressed microglia pyroptosis and motor perform restoration after SCI by delivering miR-709
As a result of the above research demonstrated that Treg-derived exosomal miR-709 will be transmitted to microglia, we questioned if miR-709 might act as a organic messenger between Treg cells and microglia, regulating microglia pyroptosis and motor perform restoration after SCI. To discover the perform of exosomal miR-709 within the Treg-Exos-regulated microglia pyroptosis after SCI, miR-709 overexpression (miR-709OE) and knockdown (miR-709KD) Treg cells utilizing a lentiviral-based technique in addition to the corresponding detrimental management (miR-NCOE and miR-NCKD) have been established.
The transfection effectivity was confirmed utilizing qRT-PCR (Fig. 6A). Exosomes have been remoted from miR-NCKD-Tregs, miR-709KD-Tregs, miR-NCOE-Tregs, and miR-709OE-Tregs named miR-NCKD-Exos, miR-709KD-Exos, miR-NCOE-Exos, and miR-709OE-Exos, respectively. A major lower within the expression of miR-709 in miR-709KD-Exos in contrast with the miR-NCKD-Exos,whereas an evident improve within the expression of miR-709 in miR-709OE-Exos when put next with the miR-NCOE-Exos was noticed (Fig. 6B). Moreover, the miR-709 expression stage within the goal BV2 microglia within the miR-709KD-Exos remedy group confirmed a dramatic lower in expression in contrast with the miR-NCKD-Exos remedy group. The 709 expression ranges within the goal BV2 microglia within the miR-709OE-Exos remedy group confirmed a rise in expression in contrast with the miR-NCOE-Exos remedy group (Fig. 6C). To evaluate pyroptosis, BV2 cells have been then handled with LPS + ATP. Fluorescent depth of GSDMD was markedly decreased by addition of miR-709OE-Exos in contrast with miR-NCOE-Exos remedy group, whereas miR-709KD-Exos remedy strongly enhanced GSDMD expression in contrast with miR-NCKD-Exos remedy group (Fig. 6D, E). Moreover, expression of pyroptosis proteins detected by western blot demonstrated related outcomes with these mentioned above (Extra file 1: Determine S4A-D).
Treg-Exos supress pyroptosis in BV2 cells by delivering miR-709 in vitro. A Transfection effectivity of miR-709 overexpression and knockdown in Treg cells; B The relative expression of miR-709 in exosomes derived from Treg cells in indicated teams; C The relative expression of miR-709 in BV2 cells administered with miR-NCOE-Exos, miR-709OE-Exos, miR-NCKD-Exos and miR-709KD-Exos; D, E Consultant immunostaining photos and quantification of GSDMD fluorescence depth in indicated teams
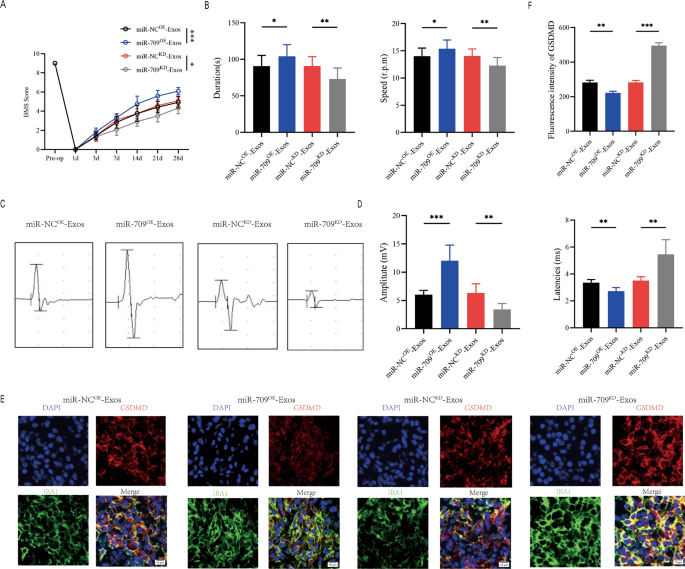
miR-709OE-Exosomes, miR-709KD-Exosomes and their detrimental management have been respectively injected WT mice instantly after SCI, and behavioral assessments have been performed on the indicated occasions. In accordance with BMS behavioral evaluation, the miR-709OE-Exosomes enhanced the impact of Treg-Exos on enhancing hindlimb motor perform following spinal wire damage, whereas miR-709KD-Exos remedy lowered the impact of Treg-Exosomes (Fig. 7A). Rotarod testing and MEPs all produced related outcomes (Fig. 7B–D). Immunofluorescence staining within the miR-709OE-Exos group in comparison with the miR-NCOE-Exos group revealed an lower fluorescence depth of GSDMD, whereas miR-709KD-Exos group in contrast miR-NCKD-Exos group exhibited an elevated fluorescence depth (Fig. 7E, F). These findings recommend that Treg-Exos, by delivering miR-709, inhibits microglia pyroptosis and promotes motor perform restoration after spinal wire damage.
Treg-Exosomes promote motor perform restoration and supress microglia pyroptosis by delivering miR-709 after SCI. A BMS was used to functionally grade mice in indicated teams as much as 28 days post-injury; B Rotarod checks evaluation of indicated teams at day 28 post-injury; C MEP evaluation was used as electrophysiological evaluation in indicated teams at Day 28 postinjury; D Quantification of peak-to-peak MEP amplitudes and latencies in indicated teams (n = 6); E Consultant immunofluorescence photos for GSDMD(pyroptosis) and IBA1(microglia) expression in indicated teams at Day 7 postinjury; F Quantification of fluorescence depth at Day 7 postinjury (n = 3)
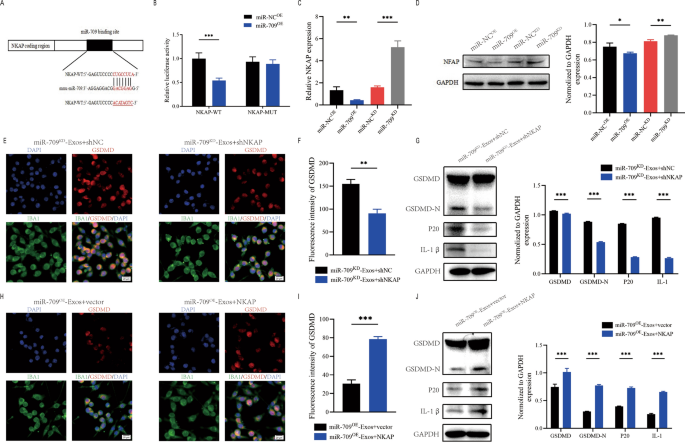
miR-709 negatively regulates NKAP
To research the underlying mechanism of motion of exosomal miR-709. In accordance with the net database of miRNA targets was used to look the expected mRNA targets for miR-709. NKAP could also be a possible goal of miR-709 (Fig. 8A). Furthermore, NKAP has been proven to play an energetic position in irritation. To verify that the NKAP 3’UTR is a direct goal of miR-709, NKAP wild-type (WT) and mutant (MUT) 3’UTR sequences have been created and cotransfected into 293T cells with miR-709 sequences.The luciferase reporter assay confirmed that miR-709 overexpression significantly lowered luciferase exercise when co-transfected with WT-3′ UTR of NKAP in comparison with management, however no inhibition exercise of miR-709 was noticed when co-transfected with MUT-3′ UTR of NKAP (Fig. 8B). Additional evaluation utilizing qRT-PCR and western blot assays confirmed that miR-709 knockdown elevated NKAP mRNA and protein ranges whereas miR-709 overexpression decreased NKAP expression(Fig. 8C, D).
Exosomal miR-709 supress microglia pyroptosis by regulating NKAP expression. A Exosomal miR-709 regulates NKAP by immediately concentrating on the three′-UTR; B Luciferase report assay was carried out to verify NKAP is the goal gene of miR-709; C The mRNA stage of NKAP in BV2 cells after remedy with miR-709OE-Exos and miR-709KD-Exos; D The protein stage of NKAP in BV2cells after remedy with miR-709OE-Exos and miR-709KD-Exos; E–G Rescue experiments for miR-709 inhibition have been performed by downregulating NKAP in microglia. microglia pyroptosis was detected by immunofuorescence (E, F) and western blot evaluation (G); H–J Rescue experiments for miR-709 overexpression have been carried out by the ectopic expression of NKAP in microglia. microglia pyroptosis was detected by immunofuorescence (H, I) and western blot evaluation (J)
The impacts of miR-709KD-Exos on microglia are restored by NKAP silencing
To additional discover the connection between exosomal miR- 709 and NKAP, some in vitro rescue trails have been carried out.Utilizing shRNA, the expression of NKAP was suppressed in BV2 cell. Outcomes revealed that inhibiting NKAP throughout co-treatment with miR-709KD-Exos lowered the activation of microglia pyroptosis (Fig. 8E, F). Furthermore, western blot supported these findings (Fig. 8G). Because of these rescue experiments, it was demonstrated that shNKAP in microglia can abolish the facilitating position of miR709KD-Exos in selling the microglia pyroptosis.
The results of miR-709OE-Exos on microglia are eradicated by overexpressing NKAP
NKAP was overexpressed by transfection with a NKAP lentivirus in microglia. Outcomes demonstrated that overexpression of NKAP promoted the activation of microglia pyroptosis throughout co-treatment with miR-709OE-Exos(Fig. 8H, I). Western blot evaluation additionally confrmed these outcomes (Fig. 8J). Subsequently, it was concluded that exosomal miR-709 suppresses the microglia pyroptosis by concentrating on NKAP.