A brand new electrocatalyst made from nickel (Ni), iron (Fe) and silicon (Si) that decreases the quantity of power required to synthesize H2 from water has been manufactured in a easy and cost-effective means, rising the practicality of H2 as a clear and renewable power of the longer term.
Hydrogen is a extremely flamable fuel that may assist the world obtain its clear power objectives if manufactured in an environmentally accountable means. The first hurdle to creating hydrogen fuel from water is the big quantity of power required for the electrolysis of water, or splitting water molecules into hydrogen fuel (H2) and oxygen (O2).
Most H2 produced right this moment is derived from fossil fuels, which contributes to world warming. Manufacturing H2 from water by means of the hydrogen evolution response (HER) requires using a catalyst, or agent that lowers the quantity of power required for a chemical response. Till lately, these catalysts had been made up of uncommon earth metals, like platinum, lowering the cost-efficiency and practicality of fresh hydrogen manufacturing.
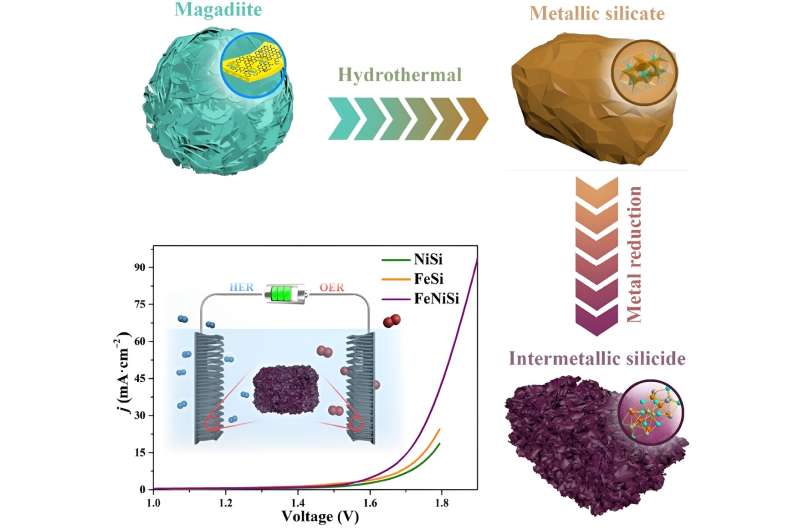
A bunch of fabric scientists from Dalian College of Know-how in Dalian, China manufactured an electrocatalyst, or a catalyst that makes use of electrical energy, utilizing cheap supplies and strategies to successfully lower the power required to generate clear H2 from water. Importantly, the ferric-nickel silicide (FeNiSi) alloy, or combination, additionally reduces the power required to generate O2 from water, making the catalyst bifunctional.
The researchers printed their research in Nano Analysis Power.
“What actually limits the event and sensible software of water electrolysis expertise is electrocatalytic supplies. At current, widespread catalysts, resembling valuable metals… are largely single-function catalysts, which limits the sensible software of water electrolysis for hydrogen manufacturing. Subsequently, the analysis and growth of environment friendly, secure, low cost and environmentally pleasant bifunctional electrocatalytic supplies is a major purpose within the discipline of electrocatalysis,” mentioned Yifu Zhang, senior writer of the research and researcher within the College of Chemistry at Dalian College of Know-how.
Transition metallic silicide alloys are distinctive compounds which can be generally utilized in energy-related fields, are cheaply produced and present promise as potential water hydrolysis electrocatalysts. These alloys are made out of transition metals, that are wonderful catalysts that freely donate and settle for electrons in chemical reactions, and Si atoms, which improve the soundness, warmth resistance and accessibility of alloy transition metallic atoms when electrical energy is utilized.
Fe and Ni, two transition metals, are well-suited to be used in a transition metallic silicide for water splitting. “Nickel silicide has been… deeply studied for its low resistance and excessive metallic exercise, particularly… in electrochemical fields. As well as, many latest research have proven that Fe-Ni primarily based supplies have appreciable potential within the discipline of electrochemical water splitting. The purpose of this work was to develop a low-cost, environmentally pleasant route to organize iron nickel silicide as a bifunctional electrolytic water catalyst (EWS),” mentioned Zhang.
The analysis staff manufactured FeNiSi in two steps. First, pure clay magadiite, a supply of silicon, iron chloride and nickel chloride had been heated underneath strain to create a ferric-nickel silicate. The ferric-nickel silicate was then mixed and heated with magnesium and sodium chloride (desk salt) to develop the ordered construction of the FeNiSi alloy. Importantly, this was the primary time a metallic silicide alloy had been manufactured utilizing this sort of chemical response utilizing metallic silicates as a response materials.
Electron microscopy and X-ray characterization strategies revealed that the manufacturing course of created many pore constructions within the last FeNiSi alloy, rising its floor space and total electrocatalytic efficiency. The FeNiSi alloy lowers the potential required to separate oxygen and hydrogen from water by 308 mV for the oxygen evolution response (OER) and 386 mV for the HER, respectively, at a present of 10 mA·cm−2. The electrocatalyst additionally demonstrated adequate sturdiness after 15 hours of use.
The analysis staff seems ahead to FeNiSi and different transition metallic silicates contributing to the synthesis of fresh hydrogen fuel for future power wants.
“This work not solely offers a straightforward methodology for the synthesis of intermetallic silicide with appreciable porous constructions but in addition permits the intermetallic silicide to be thought-about as a bifunctional electrocatalyst for EWS. Low-cost and environment friendly intermetallic silicide electrocatalysts will present new alternatives for… renewable power conversion,” mentioned Zhang.
Different contributors embrace Xuyang Jing, Yang Mu, Zhanming Gao and Xueying Dong from the College of Chemistry at Dalian College of Know-how in Dalian, China; Changgong Meng from the College of Chemistry and the School of Environmental and Chemical Engineering at Dalian College of Know-how; and Chi Huang from the School of Chemistry and Molecular Sciences at Wuhan College in Wuhan, China.
Extra info:
Xuyang Jing et al, Intermetallic ferric nickel silicide alloy derived from magadiite by magnesiothermic response as bifunctional electrocatalyst for total water splitting, Nano Analysis Power (2023). DOI: 10.26599/NRE.2023.9120104
Supplied by
Tsinghua College Press
Quotation:
Sturdy, cheap electrocatalyst generates clear hydrogen and oxygen from water (2023, November 27)
retrieved 27 November 2023
from https://phys.org/information/2023-11-durable-inexpensive-electrocatalyst-generates-hydrogen.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.


