Physicochemical properties of s-GOs and l-GOs
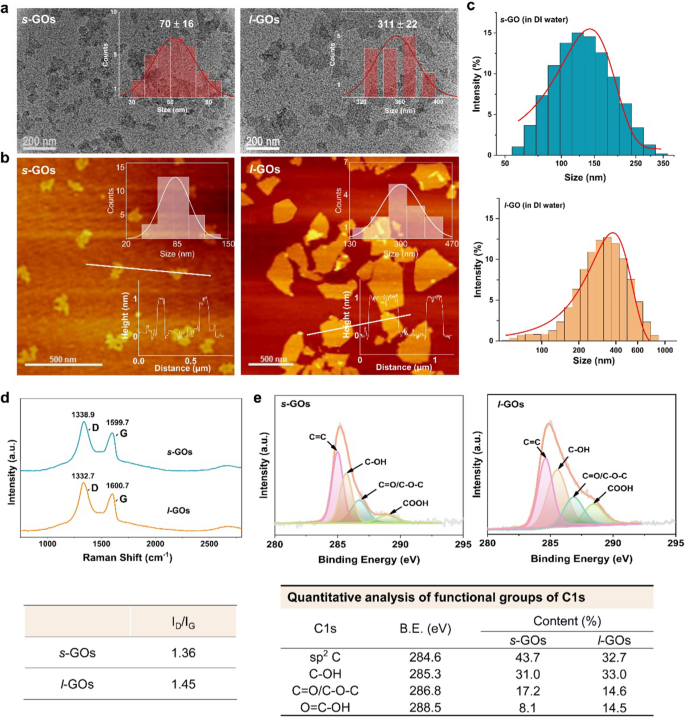
The consultant TEM pictures of GOs confirmed that each s-GOs and l-GOs had a typical flake-like construction (Fig. 1a). AFM picture evaluation exhibits the typical lateral dimension of s-GOs and l-GOs is 70 and 311 nm, respectively, and the peak is 1.02 and 1.05 nm, respectively (Fig. 1b). The dispersibility of GOs in aqueous options was assessed by hydrodynamic diameter (HD) and zeta potential measurements in deionized water. The HDs of s-GOs and l-GOs are 162 and 330 nm (Fig. 1c), respectively, that are barely bigger than the first measurement measured by AFM (Desk 1). The s-GOs and l-GOs confirmed damaging zeta potentials at − 10.3 and − 12.9 mV, respectively, indicating that the amine teams in PEG had neutralized some negatively charged carboxylic acid teams in GOs. The Raman spectrum of s-GOs consists of a typical D band at 1338.9 cm−1 and G band at 1599.7 cm−1, as for l-GOs, D band at 1332.7 cm−1 and G band at 1600.7 cm−1. The ID/IG ratios in s-GO and l-GO are calculated as 1.36 and 1.45, respectively, indicating that the GO powders have comparable oxidation diploma (Fig. 1d). Excessive decision XPS spectrum of C 1 s (Fig. 1e) present a dominant peak at 284.6 eV, which is assigned to graphitic C = C species, and the opposite three peaks at 285.7, 286.8, and 288.5 eV are equivalent to C–OH, C–O–C/C = O, and O = C–OH, respectively. Additional quantitative evaluation signifies that the s-GOs and l-GOs possess comparable floor chemistry. The labeling effectivity of Yb3+ on s-GOs and l-GOs is 35.8 and 42.3 mg/g, respectively (Desk 1). Furthermore, the Yb3+ labelled s-GOs and l-GOs confirmed very nicely dispersion stability in saline answer that greater than 99% of Yb was stably labelled on s-GOs and l-GOs for at the very least 7 days (Extra file 1: Fig. S2), which is in settlement with our earlier report [22].
Physicochemical characterization of GO nanosheets (GOs). a TEM pictures of s-GOs and l-GOs. The insert figures point out the statistical distribution of lateral measurement of GOs. b AFM pictures of s-GOs and l-GOs. The insert figures on the top-right and bottom-right point out the scale and top measurements, respectively. c Hydrodynamic measurement distributions of s-GOs and l-GOs in deionized water. d Raman spectra of s-GOs and l-GOs. e XPS spectra of s-GOs and l-GOs and quantitative evaluation of useful teams of C1s
s-GOs and l-GOs present renal clearance and urinary excretion
The GOs/ICG was used as fluorescence probe to hint the in vivo transport and clearance of GOs in mice after i.v. injection (Extra file 1: Fig. S3). The in vivo and ex vivo fluorescence detection and imaging clearly point out that s-GOs and l-GOs had comparable hepatic and renal clearance patterns (Extra file 1: Fig. S3a–c). The apparent fluorescence indicators have been noticed within the stomach at 10 min post-injection and the kidney indicators elevated from 0.5 to 1 h after which step by step decreased. At day 7 after injection, solely weak fluorescence indicators have been noticed in s-GO and l-GO handled mice (Extra file 1: Fig. S3c). The fluorescence might be noticed within the bladder (Extra file 1: Fig. S3b), suggesting the urinary excretion of GOs. The semiquantitative evaluation exhibits that in contrast with the fluorescence depth at 1 h post-injection, about 37% of s-GOs and 60% of l-GOs have been cleared at 4 h post-injection, and about 91% of s-GO and 96% of l-GO have been cleared at 24 h post-injection, indicating environment friendly renal clearance of each s-GOs and l-GOs (Extra file 1: Fig. S3d).
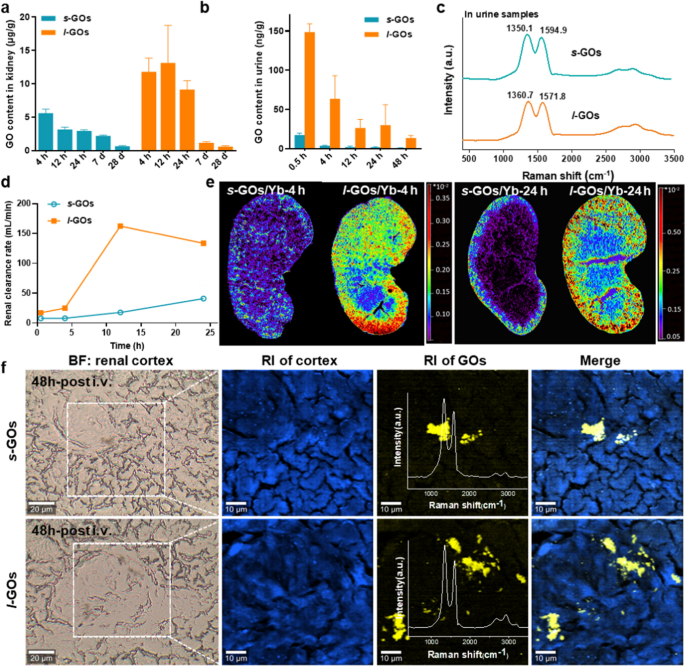
The pharmacokinetics of Yb labelling s-GOs and l-GOs (s-GOs/Yb and l-GOs/Yb) was detected by ICP-MS (Extra file 1: Fig. S4, S5). The info present that after 4 h of i.v. injection, the content material of GOs within the kidneys of l-GO handled mice was two occasions larger than that in s-GO handled mice (Fig. 2a). The renal accumulation of each s-GOs and l-GOs exhibits gradual lower from 4 h to day 28 post-injection, indicating that the GOs had been cleared from the kidneys. At day 28 after injection, the contents of GOs within the kidneys of s-GO and l-GO handled mice are comparable, solely 0.6–0.7 μg/g GOs retained (Fig. 2b), which agree with the outcomes of in vivo fluorescence imaging. The Raman signatures of GO had been detected in urine of s-GO and l-GO group mice, demonstrating the urinary excretion of intact GOs (Fig. 2c). Within the urine samples, the D and G bands appeared little wider than the first GOs, and the ID/IG ratios barely elevated to 1.58 (vs 1.36) in s-GO and to 1.65 (vs 1.45) in l-GO handled mice, suggesting the rise of the structural dysfunction of GOs throughout circulation and urinary excretion. Additional calculation of the renal clearance charge demonstrates that the renal excretion of GOs in l-GO mice was quicker than that in s-GO handled mice (Fig. 2d).
Renal clearance and urinary excretion of s-GOs and l-GOs after i.v. injection in mice. a Renal clearance patterns of s-GOs and l-GOs after i.v injection. b Urinary excretion kinetics of s-GOs and l-GOs. c Raman spectra of urine samples of s-GO and l-GO administrated mice. d Renal clearance charge of s-GOs and l-GOs. Renal clearance charge is the ratio of urinary excretion of GO ([U] × v) to plasma focus. [U]: urinary GO focus; v: urine move charge (0.002 ml/min). e LA-ICP-MS pictures of the distribution of Yb labelled GOs (s-GOs/Yb and l-GOs/Yb) within the kidney at 4 and 24 h post-injection. f Raman spectral pictures of s-GOs and l-GOs in renal cortex at 48 h after i.v. injection. From left to proper: Vivid area (BF) picture; Raman picture (RI) of autofluorescence (blue colour) or GOs (yellow colour, obtained from Raman spectrometry); merge picture of tissue and GOs
The LA-ICP-MS pictures visually show the switch and clearance pathways of GOs/Yb within the kidney parenchyma (Fig. 2e). The photographs present that the sign depth of GOs/Yb in l-GO handled mice are clearly larger than that in s-GO handled mice. At 4 h after injection, the l-GOs have been transferred from the renal artery to the renal cortex and renal medulla with the blood move and primarily distributed within the renal cortex. At 24 h post-injection, the indicators of s-GOs/Yb and l-GOs/Yb within the renal cortex and renal medulla decreased, indicating the renal clearance of GOs.
Additional, the buildup of GOs in kidneys was recognized by confocal Raman spectroscopy. The signatures of GO had been detected within the renal cortex of each s-GO and l-GO handled mice at 48 h after injection, demonstrating GO distributed within the kidneys (Fig. 2f). The ID/IG ratios within the kidney of s-GO (ID/IG ≈ 1.45) and l-GO (ID/IG ≈ 1.61) handled mouse barely elevated, in accordance with the information of urine samples. Constant outcomes have been additionally obtained by the FTIR imaging (Extra file 1: Fig. S6).
As well as, the distribution patterns of s-GOs and l-GOs in different organs present that each the s-GOs and l-GOs have been liable to accumulate in mononuclear phagocyte system (MPS) together with the liver, spleen and lung (Extra file 1: Fig. S5). Apart from, an quantity of GOs saved within the small gut, which is in accordance with the information of feces excretion in s-GO and l-GO handled mice (Extra file 1: Fig. S7). Additional, in contrast with the management, there have been no important modifications for the useful markers of the liver at 48 h and seven d post-injection of 15 mg/kg s-GOs and l-GOs to mice. (Extra file 1: Fig. S8).
Glomerular filtration and tubular secretion have been proven involving in s-GO and l-GO renal elimination
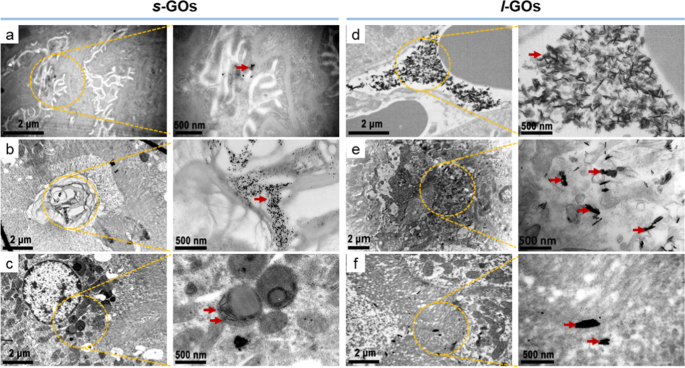
The renal clearance relies on processes of glomerular filtration, tubular secretion and reabsorption of which the tubular processes happen predominantly within the proximal tubule. We then used TEM imaging to discover the ultrastructural modifications of glomerulus and proximal tubule after s-GO and l-GO administrations. Within the s-GO handled mice, a small quantity of GOs have been discovered accumulating within the podocyte foot course of (which line the epithelial facet of the GBM), the renal tubular area and the cytosol of renal tubular epithelial cell at 4 h post-injection (Fig. 3, Extra file 1: Fig. S9), indicating that the s-GOs entered into the glomerular capsule and elements of them handed from the glomerulus into the renal tubule or in the mean time had the processes of tubular secretion and reabsorption. Whereas, within the l-GO handled mice, a substantial quantity of GOs have been noticed to build up within the peritubular capillary, tubular interstitium and tubular lumen, suggesting the method of proximal tubular secretion of l-GOs.
Ultrastructure imaging of the glomerulus and proximal tubule by TEM at 4 h after i.v. injection of s-GOs and l-GOs. s-GOs have been proven depositing in podocyte foot course of (a), the renal tubular area (b) and the cytosol of renal tubule epithelial cell (c). l-GOs deposited in peritubular capillary (d), tubular interstitium (e) and tubular lumen (f). Clusters of GOs have been indicated by crimson arrows
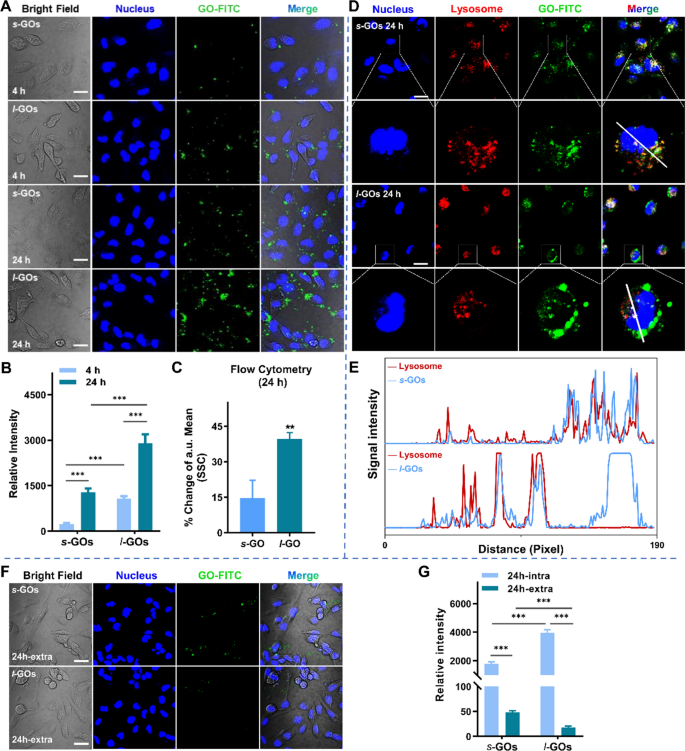
We subsequently monitor the uptake and efflux of FITC-labeled GOs (GO-FITC) in HK-2 renal proximal tubular cells utilizing confocal fluorescence microscopy. As proven in Fig. 4a, the fluorescence intensities of s-GOs/FITC and l-GOs/FITC (inexperienced) in HK-2 cells elevated with the incubation time. The quantitative evaluation signifies that the uptake of l-GOs in HK-2 cells is about fourfold and twofold larger than that of s-GOs after 4 h and 24 h coculture, respectively (Fig. 4b). Additional, move cytometry evaluation additionally demonstrated that HK-2 cells confirmed about twofold larger intracellular uptake of l-GOs/FITC than that of s-GOs/FITC counterparts at 24 h coculture (Fig. 4c). We discovered that after GOs entered into the cells, a big portion of s-GOs have been trafficked to lysosome, in distinction, a portion of l-GOs escaped the lysosome seize (Fig. 4d, f). The upper portion of l-GOs within the lysosome of HK-2 cells in comparison with that of the smaller counterparts is analogous with many earlier outcomes that the smaller sized nanoparticles are extra liable to translocate into the lysosome than the bigger sized ones [31, 32]. The efflux of s-GOs and l-GOs from HK-2 cells was additional analyzed. As proven in Fig. 4f, after GOs 24-h coculture with HK-2 cells, just a few quantities of s-GO fluorescence indicators (inexperienced) have been noticed inside the cells after 24 h exocytosis, comparatively, l-GO fluorescence have been hardly discovered, suggesting the efflux of s-GOs and l-GOs from HK-2 cells (Fig. 4g). The quantitative evaluation exhibits that the HK-2 uptake and exocytosis of l-GOs have been rather more than that of s-GOs. The CCK-8 assay exhibits that 1 ~ 200 μg/mL of s-GO or l-GO remedies induced apparent dose-dependent lower of cell viability in HK-2 cells when the doses is larger than 50 μg/mL (Extra file 1: Fig. S10). Comparatively, the l-GO therapy led extra extreme cytotoxicity than the s-GO did as a result of considerably larger inhibition of cell development was induced by l-GOs below the corresponding dose than by s-GOs.
Confocal microscopy displaying mobile uptake, localization and exocytosis of s-GOs and l-GOs in HK-2 cells. a Photographs of mobile uptake of FITC labelled (inexperienced) s-GOs and l-GOs after 4 and 24 h coculture. The nucleus was stained with DAPI (blue). b Quantitatively evaluation of uptake of s-GOs and l-GOs in HK-2 cells. c Quantitative evaluation of GO internalized in cells after 24 h therapy of s-GOs and l-GOs in comparison with untreated group by move cytometry d Subcellular colocalization evaluation of s-GOs-FITC/l-GOs-FITC and LysoTracker Pink in HK-2 cells after 24 h coculture. The nucleus was stained with DAPI (blue). The second line pictures of every GO-treated group present larger magnification of a cell. e Profiles of fluorescence sign depth alongside the straight line (white) crossing the nucleus and lysosome of a consultant s-GO/l-GO handled cell. f Exocytosis of s-GOs and l-GOs from HK-2 cells after GO-treated cells cultured in GO-free medium for twenty-four h. g Quantitatively evaluation of s-GO or l-GO exocytosis from HK-2 cells. Scale Bar: 20 μm; intra: intracellular; further: extracellular. ***p < 0.001
Perception into the expression and regulation of drug transporters in renal tubular uptake and secretion of s-GOs and l-GOs
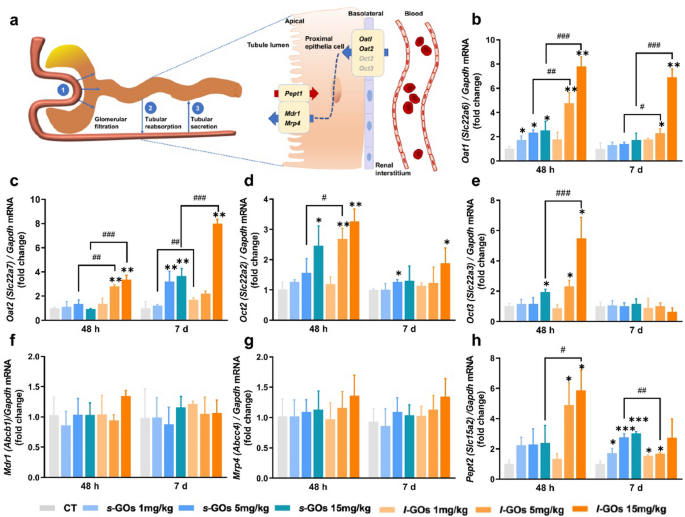
Roughly 80% renal blood flows by way of the efferent arterioles to the peritubular capillaries, the place uptake from the plasma and excretion of solutes and medicines, or vice versa, reabsorption from urine is mediated by transporters expressed on the basolateral and apical membrane of proximal tubules (Fig. 5a) [33]. The natural anion transporters (OATs) and natural cation transporters (OCTs) of the solute service 22 (SLC22) household play a serious function within the dealing with of frequent medicine and toxins [33, 34].
a A schematic diagram of the three concurrent processes of renal clearance: glomerular filtration, tubular secretion and tubular reabsorption, and the main renal tubular drug transporters concerned within the uptake and secretion of s-GOs and l-GOs. b The mRNA expression ranges b–h of renal tubular drug transporters, together with natural anion transporter 1 (Oat1/Slc22a6) and a couple of (Oat2/Slc22a7), natural cation transporter 2 (Oct2/Slc22a2) and three (Oct3/Slc22a3), ABC-transporters Mdr1 (Pgp/Abcb1) and Mrp4/Abcc4, and peptide transporter 2 (Pept2/Slc15a2) at 48 h and day 7 after i.v. injection of s-GOs and l-GOs at doses of 1, 5, 15 mg/kg bw (n = 3). *p < 0.05, **p < 0.01 and ***p < 0.001 point out the statistical distinction between handled group and the controls. #p < 0.05, ##p < 0.01 and ###p < 0.001 point out the statistical distinction between s-GO and l-GO handled teams
The transporter mRNA expression detection exhibits that the basolateral transporters of Oat1/Slc22a6, Oat2/Slc22a7, Oct2/Slc22a2 and Oct3/Slc22a3 current dose-dependent elevation within the kidneys of each s-GO and l-GO handled mice in comparison with these within the management mice at 48 h post-injection (Fig. 5b–h). Particularly, the Oat1 and Oat2 ranges within the medium (5 mg/kg) and excessive dose (15 mg/kg) l-GO handled mice elevated 2- to eightfold as in contrast with the controls and a lot of the ranges have been additionally statistically larger (p < 0.05, p < 0.001) than these within the corresponding dose of s-GO handled mice, strongly suggesting that these anion natural transporters facilitate the uptake of GOs in proximal tubular cells. Comparatively, the very best elevation of Oct2 and Oct3 was solely threefold in contrast with the controls, a lot decrease than the degrees of Oat1 and Oat2. The apically-expressed ABC-transporters: Mdr1/Pgp (Abcb1) and Mrp4/Abcc4, that are the important thing regulators for metabolism and efflux medicine [35], and peptide transporter 2 (Pept2/Slc15a2), which contributes to the tubular reabsorption, have been detected. Nonetheless, no apparent alteration of the mRNA ranges of Mdr1/Abcb1 and Mrp4/Abcc4 have been present in s-GO and l-GO handled mice. The Pept2/Slc15a2 mRNA introduced dose-dependent elevation in s-GO and l-GO handled mice at 48 h and day 7 post-injection. The very best degree was discovered roughly sixfold elevation within the excessive dose of l-GO handled mice at 48 h and threefold elevation within the s-GO handled mice at day 7 post-injection.
Kidney accidents throughout s-GO and l-GO renal excretion
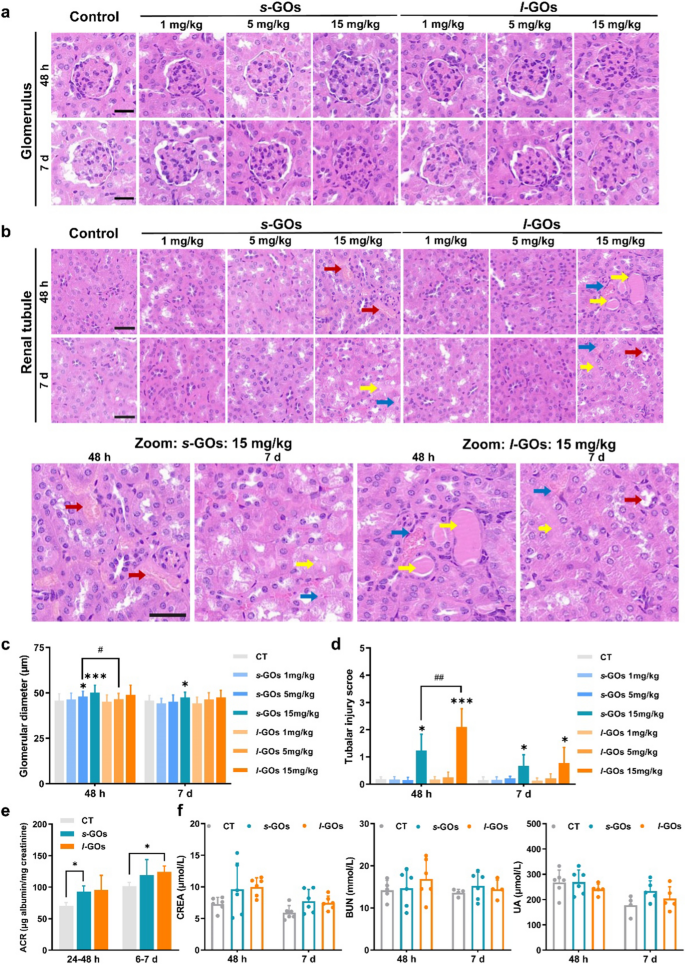
For the reason that experimental proof exhibits that the s-GO and l-GO might be excreted by the way in which of kidneys, then crucial scientific query is whether or not the processes may induce renal damage. We carried out histological examinations of the kidney at 48 h and day 7 after i.v. injection of GOs in mice (Fig. 6a, b). The glomerular diameter was noticed enhance together with injected dose within the s-GO and l-GO handled mice (Fig. 6c). No apparent pathological modifications have been noticed within the renal tubule of 1 or 5 mg/kg s-GOs and l-GOs injected mice. The apparent tubular accidents together with necrosis of renal tubular epithelial cells, forged formation and tubular dilatation have been solely noticed within the excessive dose (15 mg/kg) of s-GOs and l-GOs administrated mice (Fig. 6d), in the meantime, important enhance of glomerular diameters was noticed induced by the excessive dose of 15 mg/kg s-GO and l-GO therapy (Fig. 6c).
Histopathological examination and modifications of biomarkers of renal perform within the s-GO and l-GO administrated mice at 48 h and day 7 post-injection. a Histopathological alterations in glomerulus (n = 3), magnification: × 20. b Histopathological alterations in renal tubule (n = 3), magnification: × 20 (higher panel), × 40 (decrease panel). The crimson arrows point out necrosis of renal tubular epithelial cells; the yellow arrows point out forged formation and the blue arrows point out the tubular dilatation. c Statistical evaluation the modifications of glomerular diameter in s-GO and l-GO administrated mice. d Scoring of tubular damage. Tubular damage was outlined as necrosis, lack of brush border, forged formation and tubular dilatation. Six cortical fields of view (× 200 magnification) have been randomly chosen and the tubular damage was evaluated in line with the next scoring system: 0 = no tubular damage; 1 ≤ 10% tubules injured; 2 = 11–25% tubules injured; 3 = 26–50% tubules injured; 4 = 51–75% tubules injured; 5 > 75% tubules injured. e Measurement of 24-h urinary albumin-creatinine ratio (ACR) after injected of 15 mg/kg bw s-GOs and l-GOs to mice (n = 3). f Serum ranges of the biomarkers of renal perform after i.v. injection of 15 mg/kg s-GOs and l-GOs to mice (n = 5). CREA: creatinine; BUN: urine nitrogen; UA: uric acid. Scale Bar: 40 μm; GBM: glomerular basement membrane. *p < 0.05 and ***p < 0.001 point out the statistical distinction between handled group and the controls. #p < 0.05 and ##p < 0.01 point out the statistical distinction between s-GO and l-GO handled group
To evaluate the potential implications of renal clearance of GOs on GFB and proximal tubules, we measured the albumin-creatinine ratio (ACR) in 24-h urine samples. The ACR in each s-GO and l-GO urine samples current dose-dependent enhance at two 24-h (24–48 h and day 6–7 post-injection) excretion (Extra file 1: Fig. S11), and statistical variations have been discovered within the excessive dose of s-GO (24–48 h post-injection) and l-GO (day 6–7 post-injection) handled mouse urine (Fig. 6e). As well as, the serum biomarkers of renal perform, together with CREA, BUN and UA, introduced no apparent modifications within the s-GO and l-GO handled mice as in comparison with the management group (Fig. 6f).
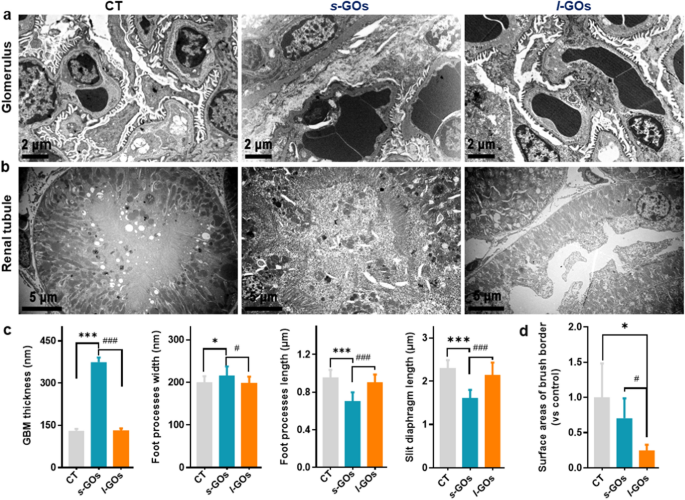
Observations of the ultrastructure of the kidney by TEM imaging additional confirmed that the excessive dose (15 mg/kg) of s-GO therapy induced apparent affect on the construction of the GFB, together with the rise of GBM thickness and podocyte foot course of width, the lower of foot course of size and podocyte slit measurement after 48 h of injection (Fig. 7a, c). The podocyte foot processes gave the impression to be diffusely effaced within the s-GO mice (Fig. 7a). Whereas, the 15 mg/kg of l-GO therapy precipitated seen tubular damage, reminiscent of renal vasodilation within the renal interstitium, renal tubule brush border loss, intratubular casts look, and epithelial cell necrosis (Fig. 7b, d).
TEM pictures of kidney ultrastructure modifications after i.v. injection of 15 mg/kg s-GOs and l-GOs at 48 h post-injection (n = 3). Ultrastructural modifications of the glomerulus (a) and renal tubular (b). c Statistical measures of GBM thickness; podocyte foot course of width; foot course of size and slit diaphragm perimeter. d Statistical measures of renal tubule brush border loss. GBM: glomerular basement membrane. *p < 0.05 and ***p < 0.001 point out the statistical distinction between handled group and the controls. #p < 0.05 and ###p < 0.001 point out the statistical distinction between s-GO and l-GO handled group
To foretell the severity of the kidney damage, we decided the mRNA expression ranges of two biomarkers of acute kidney damage (AKI): kidney damage molecule-1 (KIM1) and neutrophil gelatinase-associated lipocalin (NGAL), and a number of other inflammatory cytokines together with chemokine (C–C motif) ligand 2 (Ccl2, also called monocyte chemotactic protein-1: MCP-1), tumor necrosis issue alpha (Tnfα), interleukin-1 (IL1β) and interleukin-6 (IL6) within the kidneys (Extra file 1: Fig. S12a, b). The mRNA ranges for KIM1 and NGAL didn’t present statistic upregulation even after excessive dose of 15 mg/kg s-GO and l-GO remedies. Nonetheless, statistical elevation that greater than 1.8- to tenfold enhance of the expression ranges of Ccl2/Mcp1 and Tnfα in s-GO/l-GO kidney samples have been discovered at 48 h and day 7 post-injection. No statistical elevation of mRNA for IL1β and IL6 have been detected.