Utilizing subtle measurement strategies based mostly on near-field optical microscopy, an interdisciplinary analysis group on the Institute for Molecular Science led by Jun Nishida (Assistant Prof.) and Takashi Kumagai (Affiliate Prof.) has efficiently noticed vibrational spectra of single proteins, which consist of roughly 500 amino acid residues.
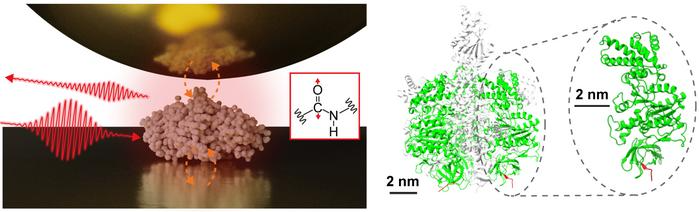
The accomplishment marks a major step ahead within the growth of technical developments comparable to ultra-sensitive and super-resolution infrared imaging, in addition to single-molecule vibrational spectroscopy.
Since infrared spectroscopy can quantify vibrational spectra, usually generally known as “molecular fingerprints,” it’s incessantly utilized for the structural and chemical investigation of a variety of supplies. Extremely-high sensitivity and super-resolution infrared imaging are in larger demand as a result of latest quick development of nanotechnology.
Nevertheless, detecting tiny samples or acquiring spatial decision on the nanoscale is past the capabilities of conventional infrared spectroscopy. It’s laborious to quantify a single protein, as an illustration, as a result of extraordinarily delicate infrared microspectroscopy wants over 1,000,000 proteins to provide an infrared spectrum.
The brand new method makes use of sunshine that’s restricted to the nanoscale, which makes it doable to investigate very small samples intimately—one thing that’s tough to do with conventional infrared spectroscopy.
The researchers used a gold substrate to isolate a single protein—a subunit of a protein advanced generally known as F1-ATPase—after which performed near-field infrared spectroscopy research in a pure setting. They made a major development by successfully acquiring the infrared vibrational spectrum of a single protein, which could assist characterize the native structural organizations of particular proteins.
With improved insights into the processes and interactions of membrane proteins and protein complexes, this info is very essential for understanding their difficult roles. Furthermore, a novel theoretical framework explaining the nanoscale interactions between the protein and the infrared close to subject has been created.
The group was in a position to statistically replicate the experimental vibrational spectra they noticed through the use of the speculation. These findings will open the door to quite a lot of makes use of for nanoscale infrared spectroscopy, together with the chemical examine of biomolecules and different nanomaterials.
Journal Reference:
Nishida, J., et. al. (2023) Sub-Tip-Radius Close to-Area Interactions in Nano-FTIR Vibrational Spectroscopy on Single Proteins. Nano Letters. doi:10.1021/acs.nanolett.3c03479.
Supply: https://www.nins.jp/en/


