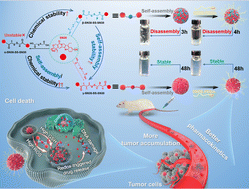
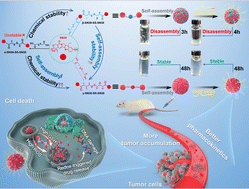
Homodimeric prodrug nanoassemblies (HDPNs) have been broadly studied for environment friendly most cancers remedy by advantage of their ultra-high drug loading and distinct nanostructure. Nevertheless, the event of SN38 HDPNs continues to be an awesome problem because of the inflexible planar fragrant ring construction. Enhancing the structural flexibility of homodimeric prodrugs by rising the linker size could also be a possible technique for setting up SN38 HDPNs. Herein, three SN38 homodimeric prodrugs with completely different linker lengths had been synthesized. The variety of carbon atoms from the disulfide bond to the adjoining ester bond is 1 (denoted as α-SN38-SS-SN38), 2 (β-SN38-SS-SN38), and three (γ-SN38-SS-SN38), respectively. Apparently, we discovered that α-SN38-SS-SN38 exhibited extraordinarily low yield and poor chemical stability. Moreover, β-SN38-SS-SN38 demonstrated appropriate chemical stability however poor self-assembly stability. As compared, γ-SN38-SS-SN38 possessed good chemical and self-assembly stability, thereby enhancing the tumor accumulation and antitumor efficacy of SN38. We developed the SN38 HDPNs for the primary time and illustrated the underlying molecular mechanism of accelerating the linker size to boost the chemical and self-assembly stability of homodimeric prodrugs. These findings would offer new insights for the rational design of HDPNs with superior efficiency.