Synthesis, in vitro response and transformation of NSBSO to GSH
The designed BSO-based and GSH-responsive peptide spinoff (NSBSO) was comprised of the next important components (Fig. 1A): (1) Nap-DFDFY, a hydrophobic peptide able to self-assembling into nanofibers; (2) DEVD-BSO, the hydrophilic peptide sequence containing BSO to regulate the steadiness of hydrophilicity and hydrophobicity and inhibit the synthesis of intracellular GSH; (3) succinic anhydride-modified cystamine as a linker of (1) and (2), and had the perform of GSH responsive cleavage, which depleted the present intracellular GSH. Particularly, we first ready the intermediates, together with Fmoc-CS, Fmoc-HDA and Fmoc-BSO, by modifying the disulfide bond linker, the management non-GSH responsive carbon–carbon bond linker and BSO with 9-fluorenylmethyloxycarbonyl (Fmoc) group and succinic anhydride by means of liquid-phase reactions (Extra file 1: Figs. S1–S3). Then, the peptide spinoff NSBSO was obtained by classical solid-phase peptide synthesis utilizing the above intermediates in line with our earlier literature [39, 40]. All of the intermediates and the ultimate product had been verified by time-of-flight mass spectrometry (TOF–MS) (Extra file 1: Figs. S4–S7). In the meantime, to research the GSH responsiveness and the perform of BSO, the management peptide spinoff Nap-DFDFY-HDA-DEVD-BSO (NCBSO) and peptide spinoff with out BSO Nap-DFDFY-CS-DEVD (NS) had been ready respectively in an identical approach (Fig. 1B and Extra file 1: Figs. S8, S9). The yields of Fmoc-CS, Fmoc-HDA and Fmoc-BSO had been 79.16%, 62.89% and 84.31%, respectively. The yields of NSBSO, NCBSO and NS had been 18.18%, 15.69% and 24.7%, respectively.
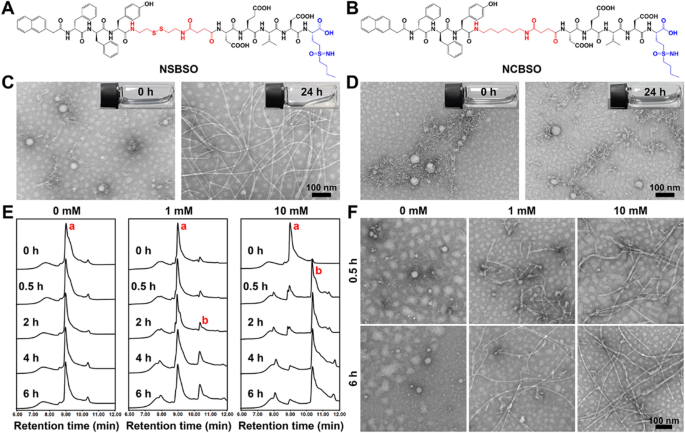
Molecular construction and in vitro response and transformation of NSBSO to GSH. A, B The molecular constructions of NSBSO and NCBSO. C, D Optical photographs (inserts) and TEM photographs of (C) NSBSO and (D) NCBSO after 10 mM GSH remedy at 37 °C for twenty-four h. E HPLC traces and F the corresponding TEM photographs of NSBSO within the presence of 0 mM, 1 mM and 10 mM GSH at 37 °C inside 6 h. The purple a and b in (E) represents NSBSO and Nap-DFDFY-thiol, respectively
Transmission electron microscopy (TEM) outcomes confirmed that NSBSO and NCBSO spontaneously assembled into spherical nanoparticles (NPs) after dissolving in phosphate buffered saline (PBS) answer with the focus of 1 mM, with the common diameter of 48.26 ± 6.17 nm and 41.27 ± 6.62 nm, respectively (Fig. 1C, D). These outcomes confirmed that NSBSO and NCBSO had related self-assembling properties. The GSH responsiveness of NSBSO and NCBSO had been validated by incubating them with GSH (10 mM) for twenty-four h. As proven in Fig. 1C inserts, the clear answer of NSBSO become sticky hydrogel after the remedy of GSH for twenty-four h, however not NCBSO (Fig. 1D inserts). The TEM outcomes had been in keeping with the visible adjustments, which illustrated the morphological transformation of NSBSO from nanoparticles to common nanofibers with diameters of ~ 10 nm and the lengths of micrometers (Fig. 1C). Whereas NCBSO nanoparticles had been steady in aqueous answer after GSH remedy (Fig. 1D).
We additional investigated the molecular adjustments of NSBSO after GSH remedy by high-performance liquid chromatography (HPLC) evaluation. When NSBSO is lowered by GSH, the disulfide bond of NSBSO will break and NSBSO will then be transformed to Nap-DFDFY-thiol and this course of could be detected by liquid chromatography-mass spectrometry (LC–MS) evaluation (Extra file 1: Fig. S10). As proven in Fig. 1E and Extra file 1: Fig. S11, NSBSO was extraordinarily steady in PBS buffer answer with out GSH and conversion from NSBSO (a) to Nap-DFDFY-thiol (b) occurred after GSH remedy. Notably, the discount of NSBSO confirmed a big dependence on the time and focus of GSH remedy. Particularly, within the presence of 1 mM GSH, NSBSO progressively transformed to Nap-DFDFY-thiol, and the conversion fee was near 25% after remedy for six h. Compared, when the focus of GSH was elevated to 10 mM, 74% of NSBSO transformed to Nap-DFDFY-thiol inside 0.5 h and the conversion fee was as excessive as 92% after 4 h remedy. As revealed in Fig. 1F, the outcomes of TEM photographs confirmed the morphological transformation of NSBSO each within the presence of 1 mM and 10 mM GSH, which was extremely in keeping with the outcomes of HPLC traces. It may be discovered that nanofibers have begun to type after remedy of 1 mM GSH for 0.5 h, which can be associated to Nap-FFY as an efficient supramolecular gelator [41, 42]. These above outcomes point out that NSBSO is extremely delicate to GSH remedy and is intently associated to the time and focus of GSH remedy. Furthermore, the outcomes point out that morphological transformation of NSBSO answer from nanoparticles to nanofibers is realized after GSH remedy. The soundness of NSBSO in 10% serum answer was analyzed. The HPLC outcomes confirmed that the soundness of NSBSO was greater than 80% after incubation for two h, and greater than 50% for twenty-four h (Extra file 1: Fig. S12). This can be as a result of the self-assembled peptides normally have higher stability after forming nanostructures than their corresponding small molecules, and the core meeting sequence of NSBSO was composed of two d-phenylalanines, which may higher resist the degradation of enzymes than l-counterparts. We additional studied the blood cell compatibility of NSBSO by hemolysis experiment. The leads to Extra file 1: Fig. S13 confirmed that NCBSO, NS and NSBSO didn’t trigger hemolysis within the focus vary of 62.5–4000 μM, indicating that every one supplies had good capability to coexist with the membrane.
GSH-dependent cytotoxicity and depletion of intracellular GSH
After validating that NSBSO is extremely delicate to GSH remedy in vitro, we explored its mobile impact induced by this molecular and morphological transformation and the simultaneous GSH regulation by measuring the cell viability in cck-8 assay. As proven in Fig. 2A and B, the calculated half-maximal inhibitory focus (IC50) values had been fully completely different for various cell traces. When the focus of NSBSO was as excessive as 100 μM, it had no apparent cytotoxicity to CT26 and NIH3T3 cells. However, notably, 24 h remedy of NSBSO confirmed sturdy cytotoxicity towards MCF-7, B16, A549 and 4T1 cells, with IC50 values of 1.074, 0.844, 1.222 and 4.741 μM, respectively, which had been a lot decrease than these of reported GSH-responsive peptide derivatives whose IC50 was higher than 100 μM [43]. What’s extra, the IC50 values of peptide derivatives covalently linked to chemotherapeutic brokers (e.g. curcumin, podophyllotoxin) had been nonetheless within the micromolar vary [44, 45].
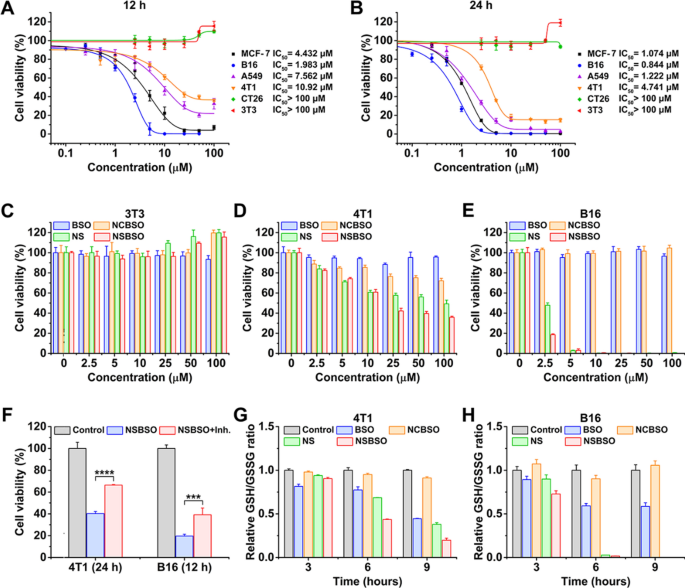
GSH-dependent cytotoxicity and depletion of intracellular GSH. A, B Cytotoxicity and IC50 values of NSBSO towards completely different cells after incubation for 12 h and 24 h. C–E Cytotoxicity of NSBSO and different formulations towards 3T3 cells, 4T1 cells and B16 cells after incubation for 12 h. F The cell viability of NSBSO in 4T1 and B16 cells w or w/o pre-treatment of GCL inhibitor (Knowledge are offered as imply ± SD, n = 4; ***P < 0.001 and ****P < 0.0001). G, H GSH/GSSG degree in 4T1 cells and B16 cells handled with 10 μM formulations for various period (Knowledge are offered as imply ± SD, n = 3)
To find out whether or not the distinction of IC50 values is said to the mobile GSH degree, we examined the intracellular GSH degree utilizing the GSH/GSSG detection equipment. As proven in Extra file 1: Fig. S14, tumor cells confirmed comparatively increased GSH than regular cells (3T3 cells) and B16 cells confirmed the best intracellular GSH degree, which had been in keeping with earlier experiences [1, 2]. Particularly, the intracellular GSH ranges from excessive to low displayed as B16 > MCF-7 > A549 > 4T1 > CT26 > 3T3, which had a optimistic correlation with the cytotoxicity of NSBSO on these cell traces (Fig. 2A, B), indicating that the cytotoxicity of NSBSO is attributable to the intracellular GSH response.
Then, we chosen B16, 4T1 and 3T3 cells as representatives of comparatively excessive, medium and low GSH ranges of cells for in-depth examine. Cells had been handled with NSBSO and management supplies (BSO, NS and NCBSO) for 12 h and 24 h and cck-8 assay was carried out. As proven in Fig. 2C and Extra file 1: Fig. S15, all formulations had no apparent cytotoxicity to 3T3 cells throughout the given focus vary. We discovered that NS and NSBSO confirmed concentration-dependent cytotoxicity in each 4T1 cells and B16 cells, which needs to be as a result of comparatively excessive degree of GSH in 4T1 cells and B16 cells (Fig. 2D, E and Extra file 1: Figs. S16, S17). Furthermore, the GSH degree of B16 cells with a stronger killing impact was 2.1 occasions increased than that of 4T1 cells (Extra file 1: Fig. S14). Nonetheless, NCBSO and free BSO, which didn’t have GSH-responsive properties, didn’t present apparent cytotoxicity throughout the given focus vary. As well as, though NS had selective cytotoxicity much like that of NSBSO, its toxicity was decrease. These variations confirmed that each the disulfide bond and BSO in NSBSO play an necessary position in killing cells.
Subsequently, to confirm the impact of various GSH ranges on NSBSO toxicity in the identical cell traces, we pretreated 4T1 and CT26 cells with completely different concentrations of GSH earlier than NSBSO remedy. The outcomes confirmed that artificially enhance the intracelluar GSH degree may improve the cytotoxicity of NSBSO (Extra file 1: Fig. S18). To additional show the affect of intracellular GSH degree on the cytotoxicity of NSBSO, we pre-treated 4T1 and B16 cells with GSH synthesis inhibitor to scale back the intracellular GSH degree, and the outcomes confirmed that the pretreatment of the inhibitors progressively lowered the intracellular GSH to a really low degree after 24 h remedy (Extra file 1: Fig. S19). However the cell morphology at 24 h after GCL inhibitor remedy didn’t change in each cells (Extra file 1: Fig. S20), which was in keeping with the outcomes reported within the literature [46]. Consequently, the addition of inhibitor remarkably compromised the cytotoxicity of NSBSO (Fig. 2F), demonstrating the cytotoxicity of NSBSO was intently associated to the excessive degree of intracellular GSH.
As well as, we evaluated the intracellular GSH degree after completely different therapies. As revealed in Fig. 2G and H, after remedy of NSBSO, the GSH/GSSG degree in 4T1 cells decreased quickly with the rise of incubation time. What’s extra noteworthy was that the GSH/GSSG degree of B16 cells decreased quickly and decreased to about 0 at 6 h, which can be as a result of GSH-responsive transformation of NSBSO in B16 cells (will likely be illustrated in Fig. 3). NSBSO quickly responded in excessive GSH B16 cells and consumed a part of GSH, after which the resultant Nap-DFDFY-thiol and NSBSO co-assembled into nanofibers within the cell, permitting extra BSO to build up, thereby inhibiting intracellular GSH synthesis and finally minimizing intracellular GSH. The extent of GSH/GSSG within the cells handled with NS for greater than 6 h was additionally considerably decreased, however the extent was smaller than that within the NSBSO group, indicating that the mix of consumption of present GSH and inhibition of GSH synthesis promotes the exhaustion of GSH in cells. Moreover, the intracellular ROS degree was detected by ROS probes. It was discovered that the intracellular ROS degree of 4T1 and B16 cells additionally elevated considerably after remedy with NSBSO or NS, and extra ROS was produced in B16 cells than in 4T1 cells (Extra file 1: Fig. S21). These outcomes point out that NSBSO has GSH-dependent cytotoxicity, which can be associated to the environment friendly depletion of GSH.
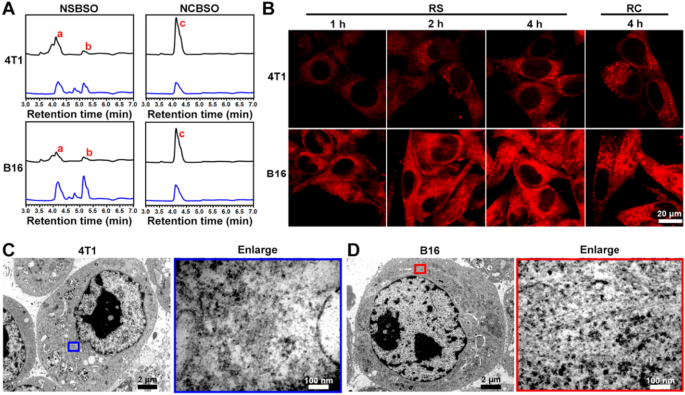
Intracellular GSH-responsive transformation. A LC–MS traces of the mobile uptake and transformation of 100 μM NSBSO and NCBSO after incubation with 4T1 and B16 cells for 10 h (black traces point out tradition medium, blue traces point out cell lysates). B CLSM photographs of 4T1 and B16 cells handled with RS or RC (100 μM). C, D Bio-TEM picture of 4T1 cell and corresponding higher-magnification picture of the blue-framed space, and B16 cell and corresponding higher-magnification picture of the red-framed space after 4-h remedy of NSBSO remedy (100 μM for 4T1 cells, 10 μM for B16 cells). The purple a, b and c in (A) represents NSBSO, Nap-DFDFY-thiol and NCBSO, respectively
Intracellular GSH-responsive transformation
The in situ meeting and morphology transformation of peptide derivatives in tumor cells have tremendously promoted the remedy of tumors. With the intention to examine the intracellular molecular transformation and the morphology transformation of NSBSO which confirmed good GSH-response in vitro and glorious selective mobile toxicity, we performed LC–MS, confocal laser scanning microscopy (CLSM) and Bio-TEM evaluation. As proven in Fig. 3A and Extra file 1: Figs. S22–25, after co-incubation with 4T1 and B16 cells for 10 h, NSBSO (a) transformed to Nap-DFDFY-thiol (b) in each cell traces, whereas NCBSO (c) didn’t bear intracellular conversion. Because of the presence of small quantity of GSH exterior the tumor cells [47], NSBSO had additionally undergone a sure diploma of extracellular conversion. It was value noting that the conversion ratio of NSBSO in B16 cells was increased than that in 4T1 cells (50% and 40%, respectively) (Extra file 1: Figs. S24, S25). We speculated that this can be associated to the upper degree of GSH in B16 cells in comparison with 4T1 cells [48]. To additional observe the transformation of peptides in residing cells, we conjugated a fluorescent indicator Rhodamine B (RhoB) to peptides and synthesized RS and RC as analogs of NSBSO and NCBSO respectively (Extra file 1: Figs. S26, S27) and carried out the mobile uptake experiment in 4T1 and B16 cells. As proven in Fig. 3B, the fluorescence depth of RS elevated with time each in 4T1 and B16 cells. Notably, RS confirmed scattered fluorescent dots inside 4T1 cells, whereas apparent filamentous aggregation in B16 cells. By comparability, though the intracellular accumulation of RC in each cells elevated over time (Extra file 1: Fig. S28), its fluorescence depth was a lot decrease than that handled with RS, nevertheless it was lower than that of RS, and a lot of the intracellular RC confirmed punctate distribution, which was considerably completely different from that of RS in B16 cells. To immediately examine the intracellular formation of molecular assemblies, we utilized Bio-TEM to picture the cells. It may be seen from Fig. 3C and D that NSBSO fashioned nanofibrous aggregates in cell plasma, particularly within the cytoplasm of B16 cells. These outcomes indicated that RS may reply to intracellular GSH and rework into fibrous nanostructure in excessive GSH degree B16 cells, which is in keeping with the outcomes of LC–MS traces (Fig. 3A) and the fast depletion of GSH in B16 cells after NSBSO remedy (Fig. 2H). Furthermore, the intracellular transformation of NSBSO and the formation of nanofibers could also be associated to its excessive cytotoxicity.
GSH-regulated tumor cell ferroptosis and mechanism examine
Based mostly on the above analysis outcomes, NSBSO confirmed a really sturdy selective killing capability of tumor cells by exhausting GSH and inhibiting GSH synthesis. Due to this fact, we subsequent studied the particular mechanism of tumor cell demise attributable to NSBSO. It has been reported that GSH depletion or the inactivation of glutathione peroxidase 4 (GPX4) can induce ferroptosis [21, 49, 50]. With the intention to confirm whether or not ferroptosis occurred in 4T1 cells and B16 cells, we pretreated cells with ferrostatin-1 (Fer-1, 0.5 μM), a ferroptosis inhibitor that forestalls the formation of lipid peroxides through a reductive mechanism [51], 24 h earlier than NSBSO remedy. As proven in Fig. 4A and Extra file 1: Figs. S29 and S30, for 4T1 cells, remedy with Fer-1 rescued NSBSO-induced cell demise by 42.13%, whereas remedy with Fer-1 didn’t markedly alleviate the NSBSO-induced B16 cells demise. Moreover, Z-VAD-FMK, an apoptosis inhibitor, scarcely rescued NSBSO-induced cell demise in 4T1 cells (Extra file 1: Fig. S31). These outcomes indicated that ferroptosis may play an necessary position in NSBSO-induced 4T1 cells demise, whereas B16 cells could have completely different demise patterns.
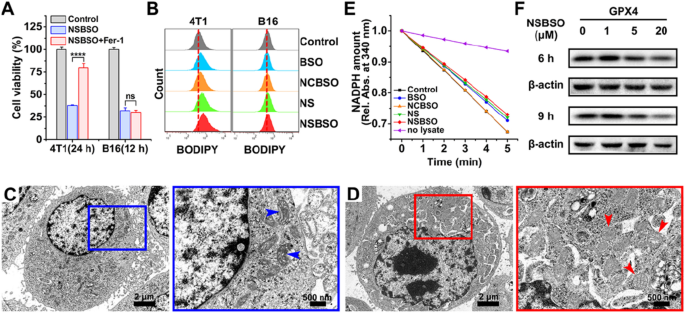
Ferroptosis of 4T1 cells induced by NSBSO and the mechanism examine. A The cell viability of 4T1 and B16 cells handled with NSBSO within the presence or absence of ferroptosis inhibitor (Fer-1, 0.5 μM) (Knowledge are offered as imply ± SD, n = 4; ****P < 0.0001). B Stream cytometry evaluation of BODIPY dye-stained 4T1 and B16 cells handled with completely different formulations (10 μM, 10 h). C, D Bio-TEM photographs of (C) an untreated 4T1 cell and (D) NSBSO handled 4T1 cell, and corresponding higher-magnification photographs of the blue and purple packing containers (arrowheads point out mitochondria). E The GPX exercise of 4T1 cells measured by the relative absorbance of NADPH discount at 340 nm (10 μM, 6 h). F The expression degree of GPX4 in 4T1 cells handled with completely different occasions and completely different concentrations of NSBSO
To additional confirm the mechanism of NSBSO-induced cell demise in 4T1 cells, lipid peroxidation and micromorphology of mitochondria had been examined. We first used BODIPY581/591 C11, a lipid peroxidation probe, to detect the lipid peroxide degree of NSBSO handled 4T1 cells. As displayed in Fig. 4B and Extra file 1: Fig. S32, the extent of lipid peroxidation in 4T1 cells elevated by 8.2% and 14% after NS and NSBSO remedy, respectively. Nonetheless, there was no important change in B16 cells after completely different therapies. We then monitored the micromorphological adjustments of 4T1 cells by Bio-TEM imaging. In contrast with untreated cells (Fig. 4C), NSBSO handled 4T1 cells exhibited shrunken mitochondria with decreased mitochondria cristae, morphological adjustments from ellipsoid to spheroid, accompanied with harm of mitochondria (Fig. 4D). These micromorphological adjustments of mitochondria had been extremely in keeping with the traits of ferroptosis [15].
As well as, the exercise and expression degree of GPX4, which performs the position of eliminating lipid peroxides and inhibiting ferroptosis, had been studied. The decreasing exercise of GPX4 in cell lysates was examined by monitoring the speed of NADPH oxidations utilizing tert-butylhydroperoxide (t-BuOOH) because the substrate. As could be seen in Fig. 4E, because the oxidation fee of NADPH (proven by the lower of absorption in 340 nm) was comparable with the management group, NCBSO remedy didn’t lower the exercise of GPX4. However the exercise of GPX4 was all suppressed to various levels in cells handled with BSO, NS and NSBSO, respectively. Notably, NSBSO remedy confirmed the strongest inhibition of GPX4 decreasing exercise. The outcomes of protein expression in western blot additionally confirmed that NSBSO remedy for 9 h considerably decreased the expression of GPX4 (Fig. 4F). The GCL degree was all the time detected by western blot. As present in Extra file 1: Fig. S33, the expression of GCL decreased in a concentration-dependent method after NSBSO remedy for 9 h. These outcomes indicated the incidence of ferroptosis in NSBSO handled 4T1 cells. As well as, for NSBSO handled B16 cells, the expression of GPX4 didn’t have apparent change, however the expression of GCL decreased clearly (Extra file 1: Figs. S34, S35), additional suggesting that NSBSO may induce ferroptosis in 4T1 cells by means of GPX4 inhibition.
Taken collectively, these findings point out that NSBSO remedy results in GSH depletion and inhibition of GSH biosynthesis, which in flip inhibits GPX4 exercise and ensuing ferroptosis in 4T1 cells. In the meantime, the depletion of intracellular GSH is accompanied by the in situ co-assembly of NSBSO and NSBSO discount merchandise, which may inhibit the biosynthesis of GSH extra effectively than free BSO which is well metabolized from cells.
GSH-regulated tumor cell pyroptosis and mechanism examine
Since Fer-1 (a ferroptosis inhibitor) did not alleviate the cytotoxicity of NSBSO towards B16 cells, we additional investigated the mechanism of the fast and intense demise of B16 cells induced by NSBSO. We famous that intracellular oxidative stress detected by 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) of B16 cells handled with NSBSO was 2.24-fold increased than that of 4T1 cells (Extra file 1: Fig. S21), which was in keeping with the outcomes that B16 cells confirmed extra important GSH depletion than 4T1 cells (Fig. 2G, H). Intracellular ROS has been lately proposed to contain in pyroptosis in tumor cells [24, 25]. Nonetheless, the particular mechanisms by which ROS participates in pyroptosis are complicated and nonetheless unclear. We hypothesized that NSBSO-induced GSH depletion triggers pyroptotic cell demise in B16 cells. To research this, we carried out cell morphology remark, lactate dehydrogenase (LDH)-release take a look at, protein degree examine and Annexin V-FITC/PI double staining evaluation.
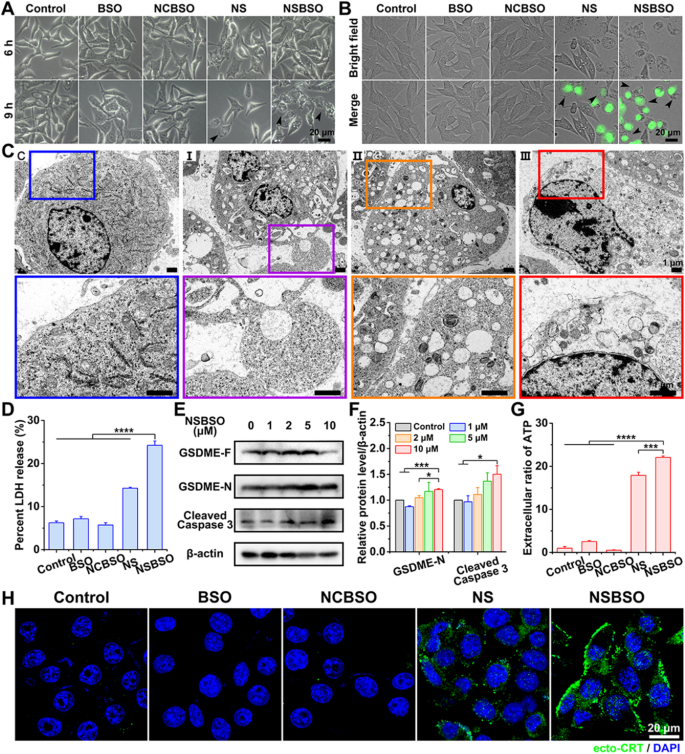
Firstly, cautious inspection of the morphology of B16 cells with numerous therapies was carried out by optical and fluorescence microscopy. As displayed in Fig. 5A, in contrast with the management group of non-treated, BSO-treated and NCBSO-treated cells, shiny spots progressively appeared on the cell floor after 6 h of NS and NSBSO remedy, and in depth shiny spots had been noticed in B16 cells handled with NSBSO for 9 h. After incubation for about 10 h, a lot of B16 cells confirmed SYTOX inexperienced staining, which is a nuclei dye of lifeless cells. In the meantime, most B16 cells confirmed clearly pyroptotic morphology, with swelling and attribute giant bubbles from the plasma membrane (Fig. 5B), indicating that pyroptosis occurred in NSBSO-treated B16 cells. In contrast, no related pyroptotic morphology was noticed in 4T1 cells handled with completely different formulations (Extra file 1: Fig. S36). The fantastic morphological adjustments of B16 cells handled with NSBSO for six h to 10 h had been additional examined through Bio-TEM, in order to confirm the pyroptosis means of B16 cells. For untreated management cells, the cell membrane was intact (Fig. 5C(C)). For NSBSO-treated cells, we discovered pore formation on cell membranes and enormous bubbles blowing from the plasma membrane (Fig. 5C(I)). We additionally noticed the rupture of bubbles (Fig. 5C(II)) and the partial and full destruction of the plasma membrane (Fig. 5C (III)). Then, the LDH launch, as a key indicator of cell pyroptosis was performed. As proven in Fig. 5D, 3.9-, 3.4- to 4.2-fold enhance of LDH launch (versus management, BSO and NCBSO, respectively) had been noticed in cells handled with NSBSO, additional validating the incidence of pyroptosis.
Pyroptosis of B16 cells induced by NSBSO and the mechanism examine. A Consultant photographs of the morphology of B16 cells handled with completely different formulations (10 μM). B Fluorescence photographs of B16 cells handled with completely different formulations (10 μM) for 10 h (SYTOX inexperienced stained nuclei of dying cells, black arrows point out cell swelling with massive bubbles). C Bio-TEM photographs of B16 cells at completely different phases after 10 μM NSBSO remedy and corresponding higher-magnification photographs of the packing containers. D LDH launch of B16 cells handled with completely different formulations for 9 h (Knowledge are offered as imply ± SD, n = 3; ****P < 0.0001). E, F The expression degree (E) and semi-quantification evaluation (F) of GSDME-N and Cleaved Caspase 3 in B16 cells handled with completely different concentrations of NSBSO for six h (Knowledge are offered as imply ± SD, n = 3; *P < 0.05 and ***P < 0.001). G Extracellular ratio of ATP of B16 cells handled with completely different formulations for 9 h (Knowledge are offered as imply ± SD, n = 3; ***P < 0.001 and ****P < 0.0001). H Immunofluorescence photographs of ecto-CRT in B16 cells handled with completely different formulations (10 μM) for 10 h
We then sought to research the mechanism of pyroptosis in NSBSO-treated B16 cells. Gasdermin D (GSDMD) and gasdermin E (GSDME), cleaved by Caspase 1 and Caspase 3 respectively, are two foremost found executors of pyroptosis [17, 52]. We discovered that Ac-DEVD-CHO, a caspase 3 inhibitor, higher rescued cytotoxicity of NSBSO towards B16 cells when evaluating with the caspase 1 inhibitor (Z-YVAD-FMK) (Extra file 1: Fig. S37). Furthermore, we detected elevated expression of cleaved caspase 3 and decreased GSDME cleavage product (GSDME-N) in NSBSO-treated cells (Fig. 5E, F). In the meantime, there was no nice distinction between the expression of Caspase 1 and GSDMD with the rise of incubation focus in NSBSO handled B16 cells (Extra file 1: Fig. S38). To confirm the important thing position of GSH response in inducing pyroptosis, we in contrast the expression of GSDMD and GSDME in 4T1, B16 and CT26 cells. We discovered that though CT26 cells had excessive ranges of GSDMD and GSDME expression (Extra file 1: Fig. S39), NSBSO remedy didn’t induce cell pyroptosis due to its low intracellular GSH degree (Fig. 2A, B and Extra file 1: Fig. S14). With the intention to additional show the position of Caspase 3 in inducing pyroptosis, we carried out Annexin V-FITC/PI double staining evaluation to differentiate pyroptosis and apoptosis in line with literature experiences [52,53,54]. The leads to Extra file 1: Fig. S40 indicated that important pyroptosis occurred in B16 cells after NSBSO remedy. Furthermore, there was no apparent early apoptotic cells, which principally dominated out the incidence of apoptosis.
Pyroptosis is usually thought of as a type of immunogenic cell demise (ICD). We detected the extracellular launch of adenosine triphosphate (ATP) from B16 cells handled with NSBSO and the leads to Fig. 5G confirmed that exceptional extracellular ATP launch was discovered after NSBSO remedy for 9 h. As well as, surface-exposed calreticulin (ecto-CRT) can also be certainly one of many molecular occasions associated to ICD. We detected the CRT publicity of B16 cells handled with NSBSO by immunofluorescence. In contrast with clean management, BSO and NCBSO teams, a small quantity of fluorescence appeared after 10 h of NS remedy, whereas NCBSO group confirmed a time-dependent enhance of fluosescence inside 10 h (Extra file 1: Fig. S41), and a considerable amount of fluorescence appeared on the cell membrane at 10 h (Fig. 5H), which indicated a considerable amount of publicity of CRT and the incidence of ICD. These outcomes counsel that NSBSO-induced pyroptotic cell demise in B16 cells is mediated by GSDME activation. Taken collectively, these findings counsel that NSBSO-induced GSH depletion and synthesis inhibition can elevate intracellular ROS, thereby activating the caspase household, resulting in GSDME cleavage and cell pyroptosis.