Supplies
MoS2 was acquired from Nanjing XFNANO Supplies Tech Co. Ltd. CS (Deacetylation diploma 90%) was obtained from Shanghai yuanye Bio-Know-how Co. Ltd. Tetrabutylammonium hydroxide (~ 25% in H2O), N-(3-Dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride (EDCI), p-toluenesulfonic acid, 3-mercaptopropionic acid, methanol, N-hydroxy succinimide (NHS), N-methylpyrrolidone (NMP), N, N-dimethylformamide (DMF), and tert-butyl nitrite had been bought from Aladdin Biochemical Know-how Co., Ltd. (China).
Mouse fibroblasts (L929), Human umbilical vein endothelial cells (HUVECs), Escherichia coli (E. coli, ATCC 25,923), and Staphylococcus aureus (S. aureus, ATCC 25,922) had been obtained from the American Sort Tradition Assortment (ATCC). Methyl thiazolyl diphenyl-tetrazolium bromide (MTT), Griess reagent package, ATP assay package, BCA Protein assay package, 3-Amino,4-aminomethyl-2’,7’-difluorescein diacetate (DAF-FM DA), and Calcein/PI cell viability/cytotoxicity assay package had been bought from beyotime biotechnology (China). Dwell/lifeless micro organism double staining package was obtained from Shanghai Beibo Biotechnology Co. Ltd. Mouse anti-CD31 and anti-VEGFα had been purchased from proteintech (China).
Preparation and characterization of MoS2 nanosheets
MoS2 nanosheets had been ready by liquid-phase exfoliation. First, bulk MoS2 was dispersed in a tetrabutylammonium hydroxide (TBAOH) answer at a focus of 5 mg mL-1 and stirred in a single day at room temperature to cut back the interlayer forces between the MoS2 sheets. Afterwards, the majority MoS2 was rinsed twice with ethanol to take away residual TBAOH. The ensuing precipitate was collected in a 50 mL centrifuge tube and dispersed in an 80% aqueous answer of NMP. The precipitate was then fully dispersed by means of bathtub sonication, and subsequently exfoliated into MoS2 nanosheets utilizing probe sonication (JY92-IIDN, Scientz) for 10 h with a 3 s on/off cycle. The answer was centrifuged for 45 min at 5000 rpm to take away any unexfoliated MoS2 particles. The resultant supernatant, containing the exfoliated nanosheets, was additional centrifuged at 15,000 rpm for 30 min to take away extra natural solvent. Lastly, the obtained precipitate was re-dispersed in deionized water, leading to a clear and homogeneous dark-green answer of MoS2 nanosheets. The construction of MoS2 nanosheets was characterised utilizing a UV-visible photometer (Ultrospec 7000, Biochrom) and Raman spectrometer (Horiba Evolution, HORIBA Scientific). The floor morphology of MoS2 nanosheets was noticed utilizing scanning electron microscopy (SEM, SU8010, HITACHI) and transmission electron microscopy (TEM, FEI Tecnai G2 F20, Thermo Fisher). The particle measurement and zeta potential of MoS2 had been analyzed utilizing a malvern particle measurement analyzer (Zetasizer Nano ZS-90, Malvern Panalytical).
Preparation and characterization of SNO-CS@MoS2
As beforehand reported, 3-(nitroso) propionic acid (SNO) was initially produced previous to the synthesis of SNO-CS [39]. Briefly, an extra of tert-butyl nitrite (4 mmol) and 212 mg 3-mercaptopropionic acid had been combined in a 2 mL answer of DMF, and the response combination was stored in an ice bathtub below a nitrogen environment for six h. Afterward, any unreacted tert-butyl nitrite was eliminated by vacuuming for 1 h to acquire the SNO answer. The distinctive peak of SNO was characterised utilizing a UV-visible photometer.
To organize natural solvent-soluble CS, p-toluene sulfonic acid and CS had been dissolved in 5 mL of deionized water at a CS focus of 10 mg mL-1. The ensuing answer was stirred in a single day at room temperature. The undissolved CS was then eliminated by centrifugation at 2000 rpm for 15 min, and the supernatant was lyophilized to acquire p-toluene sulfonic acid-modified CS. The preparation of SNO-CS primarily entails the amide condensation response between the amino group on CS and the carboxyl group on SNO. First, 1 mL SNO (1 mol mL-1), 170.5 mg EDC, and 126.5 mg NHS had been combined in 10 mL DMF, then stirred in an ice bathtub for 30 min earlier than including 50 mg p-toluene sulfonic acid-modified CS and stirring for one more 20 h in 0 ℃. The ensuing combination was then rinsed 3 times with methanol, freeze-dried, and saved at -20 °C. The SNO-CS construction was characterised utilizing an FT-IR spectrometer (ALPHAII, Bruker) and a UV-visible photometer. Completely different quantities of SNO (concentrations starting from 25, 50, 75, 100, 125.0, and 166.7 μmol L-1) and a condensation agent had been added to quantify the grafting quantity of SNO in DMF and stirred for 30 min. Equal quantities of p-toluene sulfonic acid-modified CS had been dissolved in DMF and reacted in an ice bathtub for 20 h to conduct the amidation response. The focus and normal becoming curves of SNO had been obtained utilizing the attribute peak of the SNO group within the UV-Vis spectrum at 358 nm. Primarily based on the becoming curve, the grafting effectivity of SNO on CS could be calculated, and the grafting ratio could be decided.
To organize SNO-CS@MoS2, SNO-CS was first re-dispersed in deionized water at a focus of three mg mL-1, after which progressively added into the MoS2 nanosheet dispersion (1.0 mg mL-1). The response combination was stored in an ice bathtub for 4 h. Afterwards, extra SNO-CS within the supernatant was eliminated. The precipitate was collected and dried in a vacuum drying oven to acquire a black powdery product. CS@MoS2 was ready utilizing a parallel methodology. The constructions of the SNO-CS@MoS2 and CS@MoS2 nanosheets had been characterised utilizing a UV-visible photometer and FT-IR spectrometer. The floor morphology of the SNO-CS@MoS2 nanosheets was noticed utilizing SEM and TEM. The particle measurement of SNO-CS@MoS2 and CS@MoS2, in addition to the zeta potential of SNO-CS@MoS2, CS@MoS2, SNO-CS, and CS was analyzed utilizing a Malvern particle measurement analyzer.
In vitro photothermal impact of SNO-CS@MoS2
To guage the in vitro photothermal efficiency, SNO-CS@MoS2 (200 μg mL-1) was positioned in a 2 mL plastic centrifuge tube, after which irradiated utilizing an 808 NIR laser at 0.50, 0.75, or 1.00 W cm-2 for 10 min. The temperature values and thermal photos had been recorded at 15 s intervals utilizing an infrared thermal imager digital camera (FLIR ONE Professional, TELEDYNE FLIR). Equally, the dependence of photothermal efficiency on focus was additionally examined. To calculate the photothermal conversion effectivity (η) [40], ultra-pure water and 200 μg mL-1 of SNO-CS@MoS2 had been first irradiated utilizing an 808 NIR laser at 1.0 W cm-2 for 15 min, then cooled down naturally for one more 20 min. Lastly, η was calculated utilizing the next equation:
$$eta =frac{hsleft({T}_{max}-{T}_{amb}proper)-{Q}_{0}}{Ileft(1-{10}^{{-A}_{808}}proper)}$$
(1)
$$hs=frac{m{C}_{water}}{{tau }_{s}}$$
(2)
$${tau }_{s}=-frac{t}{lntheta }$$
(3)
$$theta =frac{T-{T}_{amb}}{{T}_{max}-{T}_{amb}}$$
(4)
$${Q}_{0}=hsleft({T}_{water}-{T}_{amb}proper)$$
(5)
the place hs could be calculated utilizing Eq. (2), the place m is the mass of the SNO-CS@MoS2 answer (g), Cwater is the warmth capability of water (J/g·°C), and τs is the pattern system time fixed (s). τs could be calculated in response to method (3–4); in method (3), θ is the dimensionless driving drive, and t is the time. Furthermore, in method (4), Tmax represents the utmost steady-state temperature of the combined answer, Twater represents the utmost steady-state temperature of the water, and Tamb represents the room temperature. Q0 represents the background power enter with out a photothermal agent and is calculated utilizing Eq. (5).
To guage the photothermal stability of SNO-CS@MoS2, freshly synthesized MoS2, CS@MoS2, or SNO-CS@MoS2 dispersed in PBS had been stored at 4 °C for 5 days. Then, they underwent steady irradiation and cooling for 3 cycles utilizing an 808 nm NIR laser to evaluate the photothermal stability of those supplies.
In vitro NO launch profiles of SNO-CS@MoS2
The NO launch profiles of SNO-CS@MoS2 had been detected utilizing a Griess reagent package. Briefly, completely different concentrations of SNO-CS@MoS2 (100.0, 150.0, and 200.0 μg mL-1 in PBS) had been positioned in a 2 mL of the plastic centrifuge tube after which irradiated utilizing an 808 NIR laser at an depth of 1.0 W cm-2 for 10 min. At predetermined intervals, 100 μL of the answer was eliminated and combined with 50 μL Griess reagent. After a further half-hour of incubation at 37 °C, the quantity of NO produced was decided by measuring the absorbance of the combination at 540 nm utilizing a microplate reader (SpectroMAX M5, Molecular Units). To additional examine the self-release conduct of SNO-CS@MoS2 within the software situations, the place SNO-CS@MoS2 releases a considerable amount of NO in response to photothermal motion and exerts environment friendly antibacterial results, then residual SNO-CS@MoS2 slowly produces hint quantities of NO below physiological circumstances to advertise wound therapeutic, SNO-CS@MoS2 uncovered to 10 min of NIR mild irradiation was collected after which positioned in an incubator at 37 °C. At common intervals, 50 μL of the combination was extracted to find out the quantity of NO produced.
In vitro antibacterial property
The bacterial strains used on this research included S. aureus, methicillin-resistant S. aureus (MRSA), E. coli and extended-spectrum beta-lactamase (ESBL)-producing E. coli. Bacterial single colonies had been transferred to tryptone soy broth (TSB) liquid tradition medium and incubated in a single day at 37 °C for 12 h. The micro organism had been grown to the logarithmic stage, collected, washed, after which diluted with sterile PBS to OD600 = 0.1 for subsequent antibacterial experiments. To guage the antibacterial impact of SNO-CS@MoS2, 500 μL of micro organism was first incubated with completely different volumes of SNO-CS@MoS2 (1 mg mL-1) at 37 °C for 30 min, after which illuminated with an 808 nm laser at 1 W cm-2 density for 10 min. The bacterial suspension was then progressively diluted stepwise from 106 to 101 and uniformly inoculated onto TSB plates. After incubation at 37 °C for 18 h, the variety of bacterial colonies was counted to guage the antibacterial impact. The bacterial survival fee is expressed as log10 CFU mL-1. Micro organism handled with PBS, MoS2 (100, 200, or 400 μg mL-1), or CS@MoS2 (100, 200, or 400 μg mL-1) served as controls. SNO-CS@MoS2 (100, 200, or 400 μg mL-1) was used as a management with out NIR mild irradiation.
Dwell/lifeless staining was carried out to additional consider the antibacterial exercise of SNO-CS@MoS2. SYTO-9 and PI had been used to tell apart viable and nonviable bacterial cells. Briefly, 200 μL SNO-CS@MoS2 (1 mg mL-1) was combined with 800 μL of bacterial suspension (108 CFU mL-1) in an eppendorf tube and at 37 °C for 30 min. Then, the micro organism suspension was irradiated as described above. The micro organism had been then collected by centrifugation and stained with 100 μL of reside/lifeless backlight bacterial viability package for 30 min in the dead of night. Lastly, the stained micro organism had been washed twice with PBS and noticed below a confocal fluorescence microscope (A1, Nikon) at 60× magnification to seize fluorescent staining photos. SEM was used to look at adjustments in bacterial morphology. The micro organism had been handled as described above, then mounted with glutaraldehyde at room temperature for 4 h. After fixation, the micro organism had been washed thrice with 0.85% NaCl answer and dehydrated in an growing gradient of ethanol concentrations (20%, 40%, 60%, 80%, 90%, and 100%) in sequence for 15 min. The micro organism had been then sputtered with gold earlier than SEM examination. To quantify protein leakage from micro organism following SNO-CS@MoS2 therapy, the micro organism had been first handled as described above. The supernatant was collected to measure protein focus utilizing an enhanced BCA protein assay package and a microplate reader at 560 nm. Variations in intracellular ATP ranges in response to the SNO-CS@MoS2 remedy had been evaluated utilizing an improved ATP assay package. The micro organism had been initially handled as described beforehand, collected, and lysed with 200 μL lysis buffer. Lastly, the supernatant was collected and quantified utilizing a luminance-mode microplate reader.
Cell cultivation and in vitro cytotoxicity assay
L929 cells had been cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10% (v/v) fetal bovine serum (FBS, Gibco) and 1% (v/v) penicillin-streptomycin (P&S, Gibco) at 37 ℃, 5% CO2. HUVECs had been cultured in endothelial cell medium (ECM, Sciencell) containing 5% (v/v) FBS, 1% (v/v) progress issue (ECGS/ECGF), and 1% (v/v) P&S at 37 ℃, 5% CO2.
The cytotoxicity of SNO-CS@MoS2 in opposition to L929 cells was assessed utilizing the MTT assay. L929 cells had been seeded at a density of 5 × 103 cells per properly in 96-well plates in a single day after which handled with SNO-CS@MoS2 at 10, 20, 50, 100, 200, and 500 μg mL-1 doses for one more 24 h. Subsequent, 10 μL of MTT reagent was added to every properly and incubated for one more 4 h. After rigorously eradicating the supernatant from every properly, 150 μL of dimethyl sulfoxide (DMSO) was added, and the absorbance of every properly at 570 nm was measured utilizing a microplate reader. The reside/lifeless cell-staining assay was carried out as follows. Briefly, after 1, 3, or 5 days of incubation with SNO-CS@MoS2 (200 μg mL-1), L929 cells had been first stained with calcein-AM and PI double fluorescent stain for 30 min in the dead of night after which imaged utilizing an inverted fluorescence microscope (Axio Observer 3 supplies, ZEISS). Cells handled with PBS, SNO-CS (200 μg mL-1), and MoS2 (200 μg mL-1) served as controls.
Hemolysis assay
Complete blood was extracted from wholesome rat hearts through the use of an anticoagulant. To organize purple blood cell suspensions (RBCs), entire blood was first centrifuged, and the obtained RBCs had been then redispersed in PBS. To guage the hemolysis fee of SNO-CS@MoS2, 1 mL of SNO-CS@MoS2 at concentrations starting from 10 to 500 μg mL-1 was first combined with 20 μL of RBCs and incubated at 37 °C for 4 h. Following centrifugation at 3000 rpm for 15 min, 100 μL of the supernatant was transferred to a 96-well plate, and the absorbance of every properly was measured at 542 nm utilizing a spectrophotometric microplate reader. RBCs handled with SNO-CS and MoS2 (50–400 μg mL-1) served because the management, PBS was used as a unfavorable management, and double-distilled water was used as a constructive management.
Intracellular NO launch
The intracellular NO era conduct of SNO-CS@MoS2 within the absence of NIR irradiation was assessed as follows. Briefly, HUVECs had been inoculated onto 96-well plates at a density of 1 × 103 cells per properly in a single day. Then, 20 μL of SNO-CS-@MoS2 (1 mg mL-1) was added, and the cells had been cultured for one more 6 h. The cells had been then uncovered to 100 μL of DAF-FM DA (1 × 10− 3 M) for 30 min in the dead of night. Lastly, cells had been washed twice with PBS and noticed below an inverted fluorescence microscope. Cells handled with PBS, SNO-CS (30 μg mL-1), and MoS2 (200 μg mL-1) served because the management, SNO-CS, and MoS2 teams, respectively.
Cell scratching therapeutic
Cell scratch experiments had been carried out to evaluate the impact of SNO-CS@MoS2 on selling wound therapeutic. Briefly, L929 cells had been seeded at 1.5 × 105 cells per properly into 12-well plates and cultured to 80% confluence. The cell monolayer was then scraped with a sterile pipette tip (200 μL) and washed 3 times with PBS. Subsequently, the cells had been incubated with recent serum-free medium containing SNO-CS@MoS2 (200 μg mL-1) for six, 18, or 24 h. After that, the cells had been stained with crystal violet and noticed below an inverted microscope. Cells handled with PBS, SNO-CS (30 μg mL-1), and MoS2 (200 μg mL-1) served as management, SNO-CS and MoS2 teams.
Angiogenesis assay
To evaluate the angiogenic potential of SNO-CS@MoS2, a tube formation assay was carried out. Briefly, HUVECs (3.5 × 103) had been seeded in a single day in 48-well plates, then uncovered to SNO-CS@MoS2 (200 μg mL-1)-containing molecular mobile and developmental biology 131 (MCDB131, pricella) medium (containing 2% (v/v) FBS and 1% (v/v) P&S) for 12 h. Afterward, the cells had been digested and reseeded in 48-well plates pre-spread with matrix gel for 3 h. The cells had been then stained with calcein fluorescent dye and noticed utilizing an inverted fluorescence microscope. The entire size and the variety of junctions had been assessed through the use of the Picture J software program with an angiogenesis analyser plugin. Cells handled with PBS, SNO-CS (30 μg mL-1), and MoS2 (200 μg mL-1) served because the management, SNO-CS, and MoS2 teams, respectively. Apart from, HUVECs had been handled as described above. The cells had been then collected to measure the mRNA and protein expression ranges of CD31 and VEGF by qRT-PCR and western blotting. The primer sequences for angiogenesis-related genes (CD31 and VEGFα) are listed in Desk S1.
In vivo therapy of S. aureus-infected full-thickness cutaneous wound mannequin
All research involving animals had been reported following the ARRIVE tips and carried out in response to Nationwide Analysis Council tips for the care and use of laboratory animals. Sprague-Dawley rats (eight weeks, Male) had been obtained from Biotech Co. Ltd. (Beijing, China) and acclimatized for one week previous to the research. To ascertain the S. aureus-infected full-thickness cutaneous wound mannequin, the rats anesthetized with an intramuscular injection of ketamine (100 mg mL-1) and xylazine (20 mg mL-1) at 1 mL kg-1 of physique weight. The again of rats had been shaved, and a spherical full-thickness cutaneous wound (10 × 10 mm) space was created utilizing a punch, adopted by inoculation with 10 μL of S. aureus suspension (108 CFU mL-1) into the wound. Subsequently, all rats had been randomly divided into 5 teams (n = 8): (1) PBS + NIR, (2) SNO-CS + NIR, (3) MoS2 + NIR, (4) SNO-CS@MoS2, and (5) SNO-CS@MoS2 + NIR. The wound space was handled by direct injection of 200 μL of PBS or the supplies 3 times throughout your complete therapy interval. Rats in NIR mild irradiation group had been irradiated with an 808 nm NIR laser at 1 W cm-2 for five min. Thermal photos had been acquired by an infrared thermal imager each 50 s. On day 1, the antibacterial exercise in vivo was measured by swabbing the contaminated wound for 10 s and counting the colonies in PBS. The wound space was photographed utilizing a digital digital camera on days 0, 3, 7, 9, and 11 to find out the wound closure fee (WCR). The WCR was calculated utilizing the next equation, the place S0 represents the wound space on day 0, and S represents the wound space on the photographed day.
$$WCR left(%proper)=frac{left({S}_{0}-Sright)}{{S}_{0}} occasions 100%$$
On the finish of therapy, the rats had been sacrificed, and blood was collected utilizing a regular vein blood assortment method for hematology evaluation. The protection of the therapy course of was assessed by evaluating the variety of purple blood cells (RBC), white blood cells (WBC), hemoglobin (HGB), hematocrit (HCT), alkaline phosphatase (ALP) and aspartate aminotransferase (AST). The wound tissues and viral organs had been mounted, embedded, sectioned, and stained with hematoxylin and eosin (H&E), Masson’s trichrome, and anti-CD31 antibodies. All sections had been noticed below an inverted fluorescence microscope.
Statistical evaluation
The conventional distribution of the information was verified utilizing the Kolmogorov-Smirnov take a look at. Vital variations in these variables had been detected utilizing a one-way evaluation of variance (ANOVA). Statistical significance was set at P < 0.05.
Outcomes and dialogue
Preparation and characterization of SNO-CS@MoS2
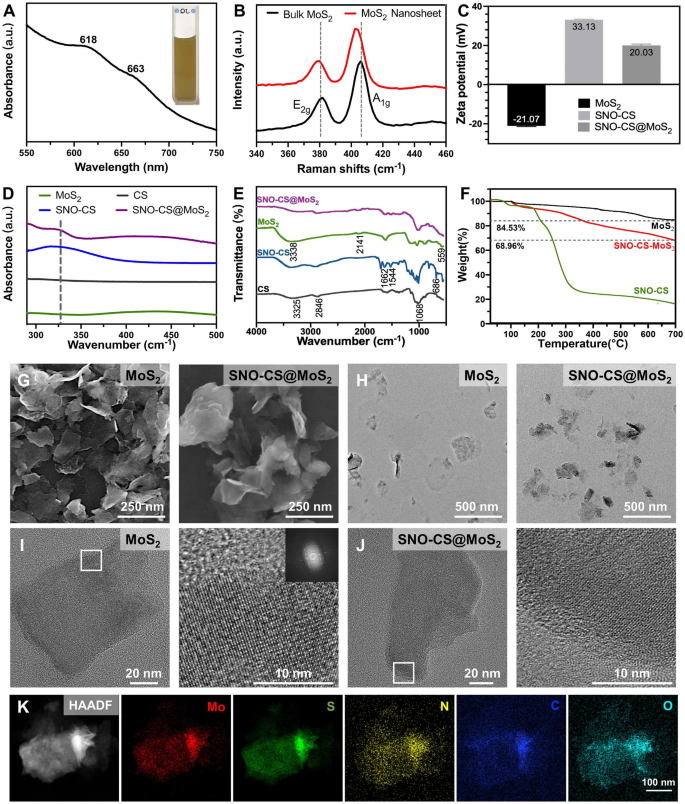
The preparation of SNO-CS@MoS2 primarily contains three steps (Scheme 1 A): 1) exfoliation of bulk MoS2 into mono- and few-layer 2D nanosheets of MoS2, (2) preparation of SNO-modified CS (SNO-CS), and (3) electrostatic adsorption of SNO-CS on the floor of MoS2 nanosheets. To organize mono- and few-layer MoS2, TBAOH was employed because the intercalation agent to cut back the van der Waals interactions between the majority MoS2 layers [41]. Subsequently, high-frequency ultrasound was employed to strip a number of layers of bulk MoS2 into mono- and few-layer MoS2 by means of acoustic cavitation [42]. The ensuing mono- and few-layer MoS2 had been then redispersed in deionized water, leading to a homogenous and clear darkish inexperienced suspension. The profitable preparation of mono- and few-layer MoS2 was confirmed by UV-vis absorption spectroscopy, morphological characterization, and Raman spectroscopy.
Determine 1 A shows the UV-vis absorption spectrum of the MoS2 nanosheet, depicting attribute absorption bands at 663 and 618 nm, which come up from the direct excitonic transitions on the Okay level of the Brillouin zone [43]. The presence of bands at 666 and 608 nm confirms the existence of mono- and few-layer MoS2, indicating the profitable exfoliation of bulk MoS2 into nanosheets [44]. The microstructure and morphology of MoS2 nanosheets had been characterised by SEM and TEM as proven in Fig. 1G-H, revealing a single or few-layered sheet-like construction with a clean floor. Determine 1B shows Raman spectra of bulk MoS2 and MoS2 nanosheets, which reveal the attribute peaks of MoS2: E12g and A1g, the place the E12g peak is the in-plane bending mode, and the A1g peak is the out-of-plane phonon mode associated largely to the stretching of the sulfur atoms [45]. It has been reported that the E12g and A1g peaks in mono- and few-layer MoS2 usually exhibit a redshift and blueshift, respectively, together with a lower in depth in comparison with that of bulk MoS2 [44]. Notably, our pattern exhibited an identical pattern, as evidenced by the E12g and A1g peaks of bulk MoS2 at 381 and 406 cm-1, respectively. In distinction, the E12g and A1g peaks of the ensuing MoS2 nanosheets shifted to 380 and 403 cm-1, respectively, and the intensities of the 2 peaks had been decreased, confirming the profitable preparation of mono- and few-layer MoS2.
The preparation of SNO-CS entails amide condensation reactions between the carboxyl teams on SNO and the amino teams on CS (Fig. S1). The UV-vis absorption and FT-IR spectra of SNO-CS confirmed the profitable synthesis of SNO-CS. It has been reported that the S-NO group has a attribute absorption band at 335–338 cm-1 in UV-vis spectrum, which is attributed to the allowed n0→ π* transition [46]. The attribute absorption band of the S-NO group could be noticed at 330–350 cm-1 within the UV-vis spectrum of SNO-CS (Fig. 1D). The synthesis of SNO-CS was additional verified by FT-IR spectra in Fig. 1E. SNO-CS reveals absorption spectra much like pure CS [47]. Moreover, within the spectra of SNO-CS, the attribute bands discovered at 1516 and 1634 cm-1 correspond to the I and II bonds of C = O, respectively. The height representing the N-H bond can be noticed within the vary of 3500–3700 cm-1, which collectively show the amide bond existence [48]. Moreover, SNO-CS additionally demonstrated a brand new peak at 686 cm-1 comparable to the -S-N = bond [30]. The above evaluation proves the profitable mixture of SNO-CS. Moreover, the grafting charges of 3-(nitroso) propionic acid on CS had been calculated utilizing the UV-vis spectra and a regular curve of 3-(nitroso) propionic acid (Fig. S2A–B). The grafting charges had been concentration-dependent, and at a focus of 125 μmol L-1, the grafting effectivity of 3-(nitroso) propionic acid was 10.31%, leading to a most grafting ratio of 15.46% (Fig. S2C). The above SNO-CS had been used for the following synthesis of SNO-CS@MoS2.
By way of electrostatic interactions, we synthesized SNO-CS-coated MoS2 (SNO-CS@MoS2) following the profitable creation of mono- and few-layer MoS2 and SNO-CS. The profitable preparation of SNO-CS@MoS2 was confirmed by the next outcomes:1) zeta potential and particle measurement distribution, 2) morphological characterization, 3) elemental mapping, 4) UV-vis absorption spectroscopy, and 5) FT-IR spectra. Intimately, the zeta potential worth charged from − 21.07 to twenty.03 mV (Fig. 1C), and the common measurement elevated from 127.5 to 341.1 nm (Fig. S3A-B) after coating of SNO-CS on MoS2. Moreover, the microstructures of MoS2 and SNO-CS@MoS2 had been characterised by SEM and TEM in Fig. 1G-H. In comparison with pure MoS2, SNO-CS@MoS2 exhibited thicker and extra uniformly dispersed nanostructures with a tough and imprecise floor. The above experiments proved that the mixture of SNO-CS and MoS2 resulted in vital adjustments within the floor morphology and roughness of the unique MoS2. By way of measurements of TEM photos, the common measurement of pure MoS2 is roughly 120 nm, which is in line with the outcomes obtained from DLS measurements. Nonetheless, the common measurement of SNO-CS@MoS2 is roughly 260 nm, which is smaller than the outcomes obtained from DLS measurements. The dimensions distinction of the SNO-CS@MoS2 measured by TEM and DLS was primarily attributed to DLS presenting moist samples. Excessive-resolution TEM was additional used to research the construction. The TEM photos confirmed a transparent lattice construction of MoS2 (Fig. 1I).The readability of the lattice construction in SNO-CS@MoS2 is decreased because of the adsorption of SNO-CS (Fig. 1J). Elemental mapping was carried out to find out the basic composition of SNO-CS@MoS2 in Fig. 1Okay and Desk S2. The outcomes confirmed the presence of Mo, S, C, N, and O, additional confirming SNO-CS loading. The above outcomes had been additional verified by the UV-vis absorption spectra in Fig. 1D and FT-IR spectra in Fig. 1E. The UV-vis absorption spectra of SNO-CS@MoS2 confirmed a shift within the absorption band of pure MoS2 from 450 to 437 cm-1 after SNO-CS modification. The attribute absorption band of the S-NO group at 330–350 cm-1 was additionally noticed. Furthermore, UV-vis-NIR absorbance spectra of MoS2 confirmed sturdy UV to NIR absorbance, which was not affected by the floor SNO-CS modification (Fig. S4). The mass extinction coefficient of MoS2 nanosheets at 800 nm was calculated to be 7.45 L g-1 cm-1, much like values measured by different researchers [49]. As well as, FT-IR spectra clearly demonstrated that SNO-CS@MoS2 had absorption spectra much like these of SNO-CS and pure MoS2. All of the above outcomes show the profitable synthesis of SNO-CS@MoS2. Moreover, the loading quantity of SNO-CS on MoS2 was estimated utilizing thermogravimetric evaluation (TGA), as demonstrated in Fig. 1F. The TGA curve of MoS2 exhibits vital weight reduction between 400 and 500 °C because of the oxidation of MoS2 to MoO3. The thermal decomposition profile of SNO-CS@MoS2 is like SNO-CS, it progressively decreases between 250 and 600 ºC, and the load loss under 150 °C could be attributed to moisture vaporization. In line with TGA knowledge, it was estimated that ~ 14% weight of SNO-CS was loaded onto the MoS2 nanosheets.
Moreover, to reveal that the synergism of SNO-CS on the PTT antibacterial exercise of MoS2 primarily originates from NO, we synthesized CS@MoS2 utilizing comparable strategies. As well as, the profitable preparation of CS@MoS2 was confirmed by particle measurement and zeta potential evaluation, as displayed in Fig. S3C-D. CS@MoS2 presents a positively charged nanostructure with a mean measurement of 212 nm. Lengthy-term stability assessments had been carried out on MoS2, CS@MoS2, and SNO-CS@MoS2. Fig. S5A-B depict images of the answer shade and particle measurement change at completely different time factors (day 1, 3, and 5) for freshly synthesized MoS2, CS@MoS2, and SNO-CS@MoS2 within the PBS answer. MoS2, CS@MoS2, and SNO-CS@MoS2 exhibited good dispersibility on day 1. Nonetheless, MoS2 aggregated on day 3, and the common particle measurement elevated to roughly 1 μm. In distinction, CS@MoS2 and SNO-CS@MoS2 exhibited excessive dispersion and stability on day 5 (Fig. S5C). This experiment demonstrated that the interlayer re-stacking of MoS2 could be inhibited after CS or SNO-CS modification, and SNO-CS@MoS2 reveals superior stability and exhibits good prospects for software in vivo.
Spectroscopic characterization of SNO-CS@MoS2 nanosheets. (A) UV-vis absorbance spectra of MoS2 nanosheets. (B) Raman spectra of bulk MoS2 and MoS2 nanosheets. (C) Zeta potential of SNO-CS, MoS2 nanosheets and SNO-CS@MoS2 nanosheets. D–E) FT-IR spectra and UV-vis spectra of CS, SNO-CS, MoS2 nanosheets and SNO-CS@MoS2 nanosheets. F) TGA curves of MoS2 nanosheets, SNO-CS and SNO-CS@MoS2 nanosheets. G–H) SEM and TEM of MoS2 nanosheets and SNO-CS@MoS2 nanosheets. I–J) Excessive-resolution TEM photos of MoS2 nanosheets and SNO-CS@MoS2 nanosheets. Okay) Elemental mappings of Mo, S, C, N and O of SNO-CS@MoS2 nanosheets
Photothermal efficiency and NO era conduct
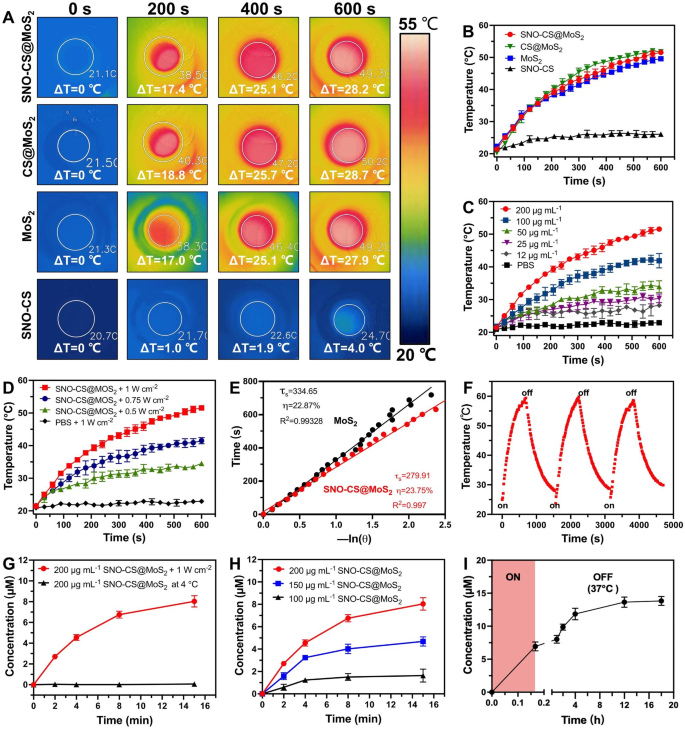
After efficiently getting ready SNO-CS@MoS2, we systematically evaluated the photothermal properties of SNO-CS@MoS2. Determine 2 A-B show the temperature enhance of SNO-CS@MoS2, CS@MoS2, MoS2, and SNO-CS below 808 nm NIR mild irradiation. The outcomes reveal that MoS2 has glorious photothermal conversion efficiency, and the coatings of CS and SNO-CS didn’t have an effect on the photothermal efficiency of MoS2. Research have demonstrated that temperatures over 50 °C kill micro organism by inactivating enzymes associated to bacterial life actions [3]. The temperature of SNO-CS@MoS2 answer elevated by 25 °C after 6 min of irradiation, indicating that it might obtain efficient antibacterial efficiency in vivo. As well as, the leads to Fig. 1C-D present that the photothermal properties of SNO-CS@MoS2 nanosheets rely upon the focus and laser depth. Underneath a set laser energy (1.0 W cm-2), the answer is heated with growing concentrations. Furthermore, the answer temperature elevated with the laser depth at a set focus (200 μg mL-1). The photothermal conversion effectivity (PCE) of the freshly ready nanomaterials is calculated in Fig. 2E and S6C. The PCE of SNO-CS@MoS2 was 23.75%, barely much like that of CS@MoS2 (24.37%) and MoS2 (22.87%), and is expounded to the excessive photothermal stability of freshly ready MoS2. The long-term photothermal stability of SNO-CS@MoS2 was additionally investigated. After 5 days of setting, three heating/cooling cycles below 808 nm radiation had been utilized to MoS2, CS@MoS2, and SNO-CS@MoS2 (Fig. 2F and S6A-B). The temperature of pure MoS2 reached solely 34 °C after 10 min of irradiation, which was a lot decrease than that on the primary day (49.57 °C). In distinction, CS@MoS2 and SNO-CS@MoS2 exhibit excessive photothermal stability throughout all three switching cycles. This outcome confirmed that the long-term photothermal stability of MoS2 nanosheets was vastly improved after chitosan or SNO-CS modification. In conclusion, the above investigations depicted that SNO-CS@MoS2 possesses glorious photothermal traits and is a promising photothermal agent for each in vivo and that in vitro purposes.
After demonstrating the wonderful photothermal efficiency of SNO-CS@MoS2, we additional evaluated the NO-releasing conduct of SNO-CS@MoS2 utilizing the Griess assay. Quantitative NO investigation was carried out utilizing a regular curve of NO (Fig. S7). Determine 2G depicts that below 808 nm NIR irradiation (1 W cm− 2), the quantity of NO generated by SNO-CS@MoS2 was a lot larger than that saved at 4 °C, indicating efficient management of NO launch by NIR laser irradiation. Moreover, NO launch from the SNO-CS@MoS2 nanosheets was concentration-dependent (Fig. 2H). The quantity of launched NO elevated with larger concentrations. Amongst them, 200 μg mL− 1 of SNO-CS@MoS2 can launch practically 7 μmol L− 1 of NO after 8 min of NIR irradiation, totally assembly the efficient antibacterial focus necessities.
Along with the environment friendly launch of NO by laser irradiation, we additional demonstrated that SNO-CS@MoS2 can produce low concentrations of NO in a physiological setting. To detect the NO launch below the physiological setting, we positioned RSNO, SNO-CS and SNO-CS@MoS2 in PBS below pure mild at 37 ℃. The outcomes confirmed that the NO launch from the three teams was mainly the identical, indicating that the gradual launch of RSNO was primarily associated to pure mild and room temperature [50, 51] (Fig. S8). Moreover, we collected the SNO-CS@MoS2 answer after 10 min of irradiation and monitored its long-term NO-release conduct at 37 °C. As illustrated in Fig. 2I, SNO-CS@MoS2 can launch low concentrations of NO after the removing of NIR irradiation, and the elevated quantity of NO might be cumulative to six.88 μmol L− 1 from 10 min to 18 h. In conclusion, the above experiments have demonstrated that SNO-CS@MoS2 nanosheets possess the power to generate NO below photothermal management and exhibit sustained NO era in a physiological setting.
The photothermal impact and NO era conduct of SNO-CS@MoS2 nanosheets. (A) Infrared thermal photos of SNO-CS, MoS2, CS@MoS2 and SNO-CS@MoS2 (focus: [MoS2] = 200 μg mL-1, [SNO-CS] = 30 μg mL-1) below NIR irradiation (808 nm, 1 W cm-2). and (B) corresponding temperature profiles. (C) Temperature profiles of SNO-CS@MoS2 in numerous focus (12, 25, 50, 100 and 200 μg mL-1) below NIR irradiation (808 nm, 1 W cm-2). (D) Temperature profiles of SNO-CS@MoS2 at completely different mild depth below an 808 nm laser (0.5, 0.75 and 1 W cm-2). (E) The cooling time plot versus − ln(𝜃) of MoS2 and SNO-CS@MoS2 nanosheets (200 μg mL-1). (F) Temperature elevations of SNO-CS@MoS2 nanosheets for 3 NIR mild irradiations cycles below an 808 nm laser (200 μg mL-1, 1 W cm-2). (G) NO launch curves of SNO-CS@MoS2 nanosheets below NIR irradiation (808 nm, 1 W cm-2) or saved at 4 °C. (H) NO launch curves of SNO-CS@MoS2 nanosheets in numerous focus (100, 150 and 200 μg mL-1) below NIR irradiation (808 nm, 1 W cm-2). (I) Accumulation NO launch inside 18 h on SNO-CS@MoS2 nanosheets after 10 min of irradiation (808 nm, 1 W cm-2)
In vitro antibacterial results of SNO-CS@MoS2
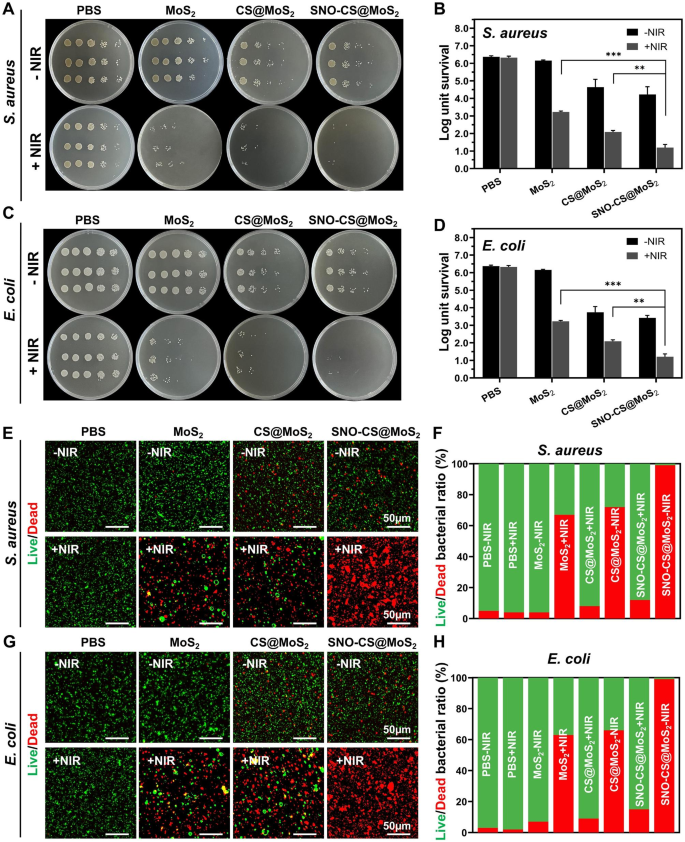
The above research have demonstrated the photothermal property and NO-producing functionality of SNO-CS@MoS2, which prompted additional investigation into its antibacterial properties. The antibacterial experiment in opposition to Gram-positive S. aureus and Gram-negative E. coli was carried out utilizing the plate counting methodology. As displayed in Fig. 3A-D, within the absence of a NIR laser, the PBS and MoS2 therapy teams exhibited no antibacterial impact, whereas the CS@MoS2 and SNO-CS@MoS2 teams demonstrated some antibacterial impact, which can be attributed to the inherent antibacterial property of CS [52]. After 10 min of publicity to NIR irradiation, the survival fee of micro organism within the MoS2 and CS@MoS2 teams displayed a big downward pattern in contrast with the corresponding teams with out irradiation therapy. The CS@MoS2 + NIR group exhibited log reductions of three.29 for S. aureus and 1.92 for E. coli, indicating that the native hyperthermia produced by PTT of MoS2 exhibited excessive antibacterial results. The SNO-CS@MoS2 + NIR exerted the best antibacterial impact. Moreover, the bacterial colonies of S. aureus and E. coli considerably decreased by 4.07 and 4.71 log, respectively, demonstrating {that a} excessive focus of NO considerably enhances the antibacterial impact of PTT. Furthermore, the synergistic impact of NO on MoS2 PTT antibacterial remedy was concentration-dependent. As displayed in Fig. S9–10, the synergistic impact of SNO-CS@MoS2 was extra apparent at low concentrations (100 μg mL− 1) whereas inconspicuous at excessive concentrations (400 μg mL− 1). Furthermore, a rise within the focus of SNO-CS@MoS2 led to larger PTT temperatures, posing a threat of tissue harm. Due to this fact, we chosen 200 μg mL− 1 as the ultimate focus. The reside/lifeless staining method confirmed the synergistic antibacterial motion. The proportion of dwelling and lifeless micro organism could be straight noticed within the fluorescence photos (Fig. 3E-H). Solely purple fluorescent spots had been noticed in S. aureus and E. coli of the SNO-CS@MoS2 + NIR group, implying that SNO-CS@MoS2 therapy with NIR killed virtually all micro organism. Intense inexperienced fluorescence was nonetheless noticed in MoS2 + NIR and CS@MoS2 + NIR teams. This analysis additional demonstrates the feasibility and efficacy of the synergistic antibacterial exercise of NO and PTT. Moreover, the principle challenge with antibacterial brokers is bacterial multidrug resistance. We additional carried out in vitro antibacterial experiments in opposition to resistant micro organism utilizing plate counting methodology. The outcomes are typically align with the findings talked about above (Fig. S11).
Antibacterial impact of SNO-CS@MoS2. (A) Images of bacterial colonies fashioned by S. aureus after varied therapy; (B) The corresponding bacterial viabilities of S. aureus; (C) Images of bacterial colonies fashioned by E. coli after varied therapy; (D) The corresponding bacterial viabilities of E. coli; E–F) Fluorescent photos of S. aureus and corresponding statistical knowledge; G–H) Fluorescent photos of E. coli and corresponding statistical knowledge. (Laser: 808 nm, 1.0 W cm-2, 10 min; Focus: 200 μg mL-1)
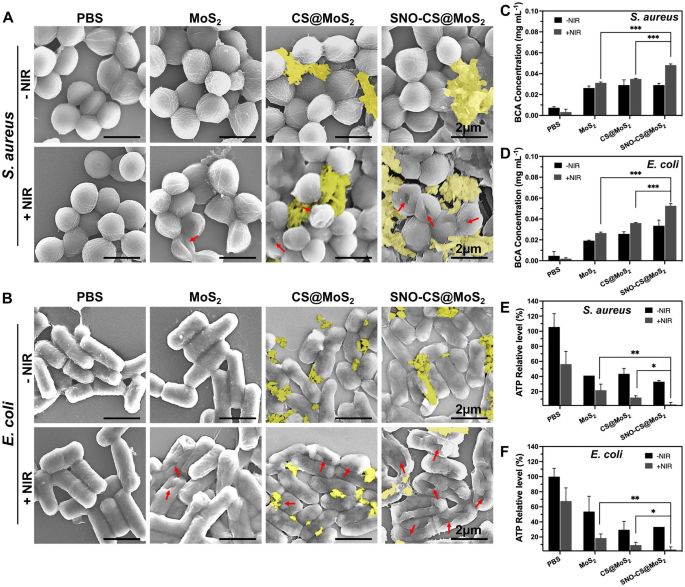
After confirming the wonderful synergistic antibacterial efficiency of SNO-CS@MoS2, experiments had been carried out to additional examine the antibacterial mechanisms. Firstly, adjustments within the morphology and membrane integrity of S. aureus and E. coli after SNO-CS@MoS2 therapy had been noticed utilizing SEM. As demonstrated in Fig. 4A-B, within the presence or absence of NIR laser, each S. aureus and E. coli within the PBS therapy teams exhibited intact spherical morphology, attribute rod-like constructions, clean surfaces, and intact cell membrane constructions. Comparatively, MoS2 + NIR and CS@MoS2 + NIR precipitated various levels of membrane contraction and deformation. In distinction, therapy with SNO-CS@MoS2 + NIR resulted probably the most pronounced deformation and lack of cell integrity in S. aureus and E. coli (proven by purple arrows), resulting in micro organism loss of life owing to the discharge of their contents. Furthermore, the presence of nanomaterials hooked up to the bacterial floor (yellow overlay) was noticed within the CS@MoS2 and SNO-CS@MoS2 teams. Primarily based on these findings, we speculate that CS@MoS2 and SNO-CS@MoS2 can adsorbed onto micro organism by means of electrostatic interactions and harm the bacterial membrane by the bodily impact of PTT and NO. The rupture of bacterial cell membranes additional results in huge protein leakage from the micro organism. As illustrated in Fig. 4C-D, the quantity of protein launched from S. aureus and E. coli within the SNO-CS@MoS2 + NIR group was roughly 1.4- and 1.5-fold larger than that within the different therapy teams, respectively. Extreme breach of the bacterial cell membrane leads to the lack of cardio respiration-related enzymes on the cell membrane, thereby blocking intracellular ATP era. The manufacturing of ATP of S. aureus and E. coli decreased by 97.19 and 96.57% after SNO-CS@MoS2 therapy with NIR irradiation (Fig. 4E-F). In conclusion, the above outcomes point out a big synergistic antibacterial impact between PTT and NO. The potential antibacterial mechanism could be attributed to the next:1) the adhesion functionality of the positively charged SNO-CS@MoS2; 2) the mixture of the reactive byproducts of NO and bodily harm from PTT, resulting in the bacterial cell membrane rupture, substantial protein leakage and inhibition of intracellular ATP synthesis; and three) NO byproducts might induce bacterial cell loss of life by means of mechanisms equivalent to DNA harm and protein dysfunction.
Antibacterial mechanisms of SNO-CS@MoS2 nanosheets. A–B) SEM photos of S. aureus and E. coli. Pink arrows marked the damaged websites of micro organism. yellow mark coated the nanosheets. C–D) the quantity of protein launched from S. aureus and E. coli following receiving varied therapies. E–F) Modifications of intracellular ATP synthesis in S. aureus and E. coli after varied therapies. (Laser: 808 nm, 1.0 W cm-2, 10 min; Focus: 200 μg mL-1)
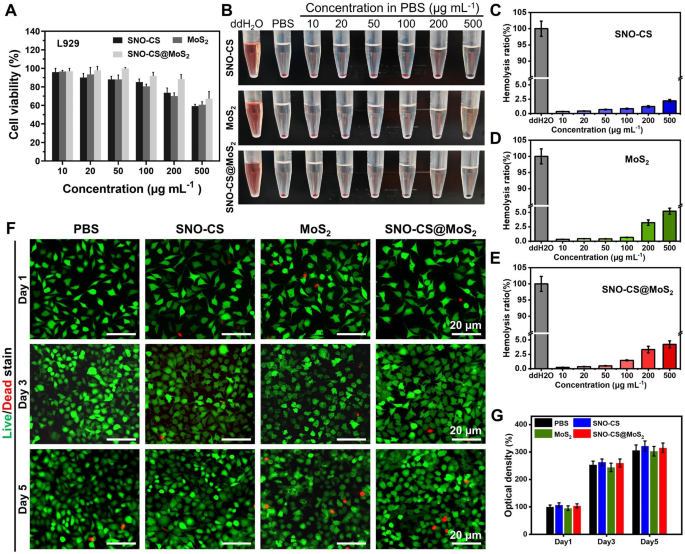
Biocompatibility assay of SNO-CS@MoS2
The biocompatibility of SNO-CS@MoS2 is crucial for its subsequent organic purposes. The cytotoxicity of SNO-CS@MoS2 in fibroblasts (L929 cells) was evaluated utilizing the MTT assay and the hemolysis take a look at. The biocompatibility after 24 h of co-incubation with completely different concentrations of nanomaterials was quantified in Fig. 5A by MTT assay. Under or equal to 200 μg mL− 1, over 85% of L929 cells survived after incubation with SNO-CS@MoS2, larger than MoS2 alone. This enchancment in biocompatibility could be attributed to the encapsulation of SNO-CS. As demonstrated in Fig. 5B, insignificant hemolysis was noticed in all therapy teams, and the quantification outcomes depicted that the hemolysis fee was decrease than 5% (Fig. 5C-E). Contemplating the antibacterial take a look at outcomes and biocompatibility findings, a focus of 200 μg/mL of nanomaterials was chosen for subsequent experiments. To watch the long-term mobile biocompatibility of the supplies, reside/lifeless staining was used. Determine 5 F shows fluorescence photos of reside/lifeless staining of L929 cells after co-incubation with completely different nanomaterials for 1, 3, and 5 days. All therapy teams demonstrated low cytotoxicity, with just a few lifeless L929 cells (purple fluorescence). Moreover, because the incubation interval elevated, the SNO-CS and SNO-CS@MoS2 teams exhibited a barely extra proliferative pattern in comparison with the opposite teams.
Biocompatibility and blood compatibility of SNO-CS@MoS2 nanosheets. A) MTT assay of various therapies in varied focus (10, 20, 50, 100, 200 and 500 μg mL-1). B–E) Hemolysis images and hemolysis ratio (%) of various therapies in varied focus (10, 20, 50, 100, 200 and 500 μg mL-1). F–G) Dwell/lifeless staining of L929 cells at 1, 3, and 5 d after handled with PBS, SNO-CS, MoS2, and SNO-CS@MoS2. (Focus: [MoS2] = 200 μg mL-1, [SNO-CS] = 30 μg mL-1)
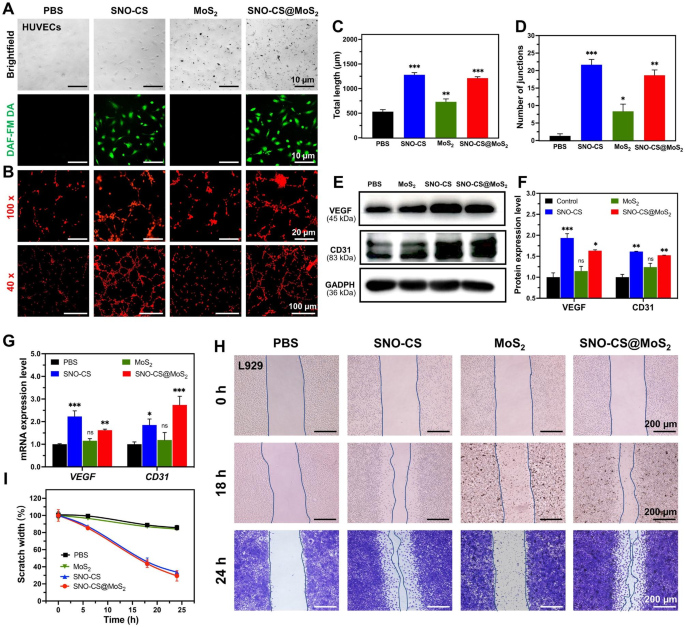
SNO-CS@MoS2 selling angiogenesis and cell scratch therapeutic
Along with effectively killing micro organism within the contaminated wound space, selling speedy wound therapeutic can be a key side of efficient wound care. The power of low concentrations of NO to advertise wound therapeutic has been documented and confirmed to play an essential function in selling vascular regeneration and epithelial cell migration. First, DAF-FM DA was utilized to trace intracellular NO launch. As displayed in Fig. 6A, after 12 h of co-culture, HUVECs within the SNO-CS and SNO-CS@MoS2 teams confirmed a powerful inexperienced fluorescence sign than the PBS and MoS2 teams. It signifies that SNO-CS@MoS2 might progressively produce hint quantities of NO intracellularly below physiological circumstances. Due to this fact, we evaluated its pro-angiogenic and pro-wound-healing properties. Determine 6B depicts that higher tube formation was noticed after 3 h of incubation with SNO-CS@MoS2 and SNO-CS, in sharp distinction to the PBS- and MoS2-treated teams. The variety of nodes and the overall size of the fashioned vessels had been calculated in Fig. 6C–D utilizing Picture J software program. These calculation parameters had been considerably larger for the SNO-CS and SNO-CS@MoS2 teams in comparison with the PBS and MoS2 teams. In conclusion, the above outcomes reveal that hint quantities of NO slowly launched from SNO-CS@MoS2 can promote angiogenesis.
Vascular endothelial progress issue (VEGF) is a vital protein concerned in angiogenesis. At the moment, research demonstrated that NO participates within the sign transduction strategy of VEGF-induced angiogenesis in vitro and in vivo [53]. Briefly, hint quantities of NO can mediate HIF-1 and HO-1 activation through the PI3K-Akt pathway, thereby upregulating VEGF expression [54]. Platelet endothelial cell adhesion molecule (CD31) is a crucial marker of vascular endothelial differentiation and is important in vascular growth by sustaining vascular perform [55]. These indicators are generally used to evaluate angiogenesis. Due to this fact, qRT-PCR and western blotting had been used to verify the angiogenic mechanism of SNO-CS@MoS2. Determine 6E-G depict VEGF and CD31 expression in HUVECs after co-culture with completely different supplies for twenty-four h. The protein and mRNA expression ranges of VEGF and CD31 had been considerably larger within the SNO-CS and SNO-CS@MoS2 teams than within the PBS and MoS2 teams. These findings counsel that SNO-CS@MoS2 can effectively launch hint NO to activate VEGF and CD31 expression, thereby growing vascular formation.
Within the center and ultimate phases of wound therapeutic, tissue reparative cells, equivalent to epidermal cells and fibroblasts, migrate and proliferate in direction of the wound tissue to advertise wound closure, which is a vital physiological response within the wound therapeutic course of. Research have proven that NO is essential for regulating wound epithelization and may promote the proliferation and migration of fibroblasts close to the wound space [56]. Primarily based on this, we subsequent investigated the power of NO to advertise fibroblast migration utilizing scratch experiments. As displayed in Fig. 6H-I, after the co-incubation for twenty-four h, the scratch therapeutic charges of L929 cells within the SNO-CS and SNO-CS@MoS2 teams had been noticeably larger in comparison with PBS and MoS2. The scratch therapeutic fee of SNO-CS@MoS2 (70.64%) was a lot larger than PBS (14.25%). The outcomes of the scratch experiment, together with the reside/lifeless labeling of L929 cells, demonstrated that SNO-CS@MoS2 can improve the migration and proliferation of L929 cells to the wound by producing hint quantities of NO on the contaminated wound website. In abstract, SNO-CS@MoS2 can promote angiogenesis and the migration and proliferation of tissue restore cells to the wound by slowly producing a small quantity of NO within the cells, thus considerably accelerating the contaminated wound therapeutic.
Mechanisms of SNO-CS@MoS2 selling wound therapeutic. (A) Brightfield and fluorescent picture of HUVECs after handled with PBS, SNO-CS, MoS2, and SNO-CS@MoS2. DAF-FM DA used as NO probe; (B) Tube formation assay of HUVECs after varied therapy; C–D) Quantitative evaluation of the variations within the variety of nodes and complete size between teams. E–F) Protein expression ranges and quantitative evaluation of VEGF and CD31 in HUVECs after varied therapy. G) mRNA expression ranges of VEGF and CD31 in HUVECs after varied therapy. H–I) Photographs and quantitative evaluation of cell migration of L929 cells after handled with PBS, SNO-CS, MoS2, and SNO-CS@MoS2. (Focus: [MoS2] = 200 μg mL-1, [SNO-CS] = 30 μg mL-1)
The efficiency of selling wound therapeutic in vivo
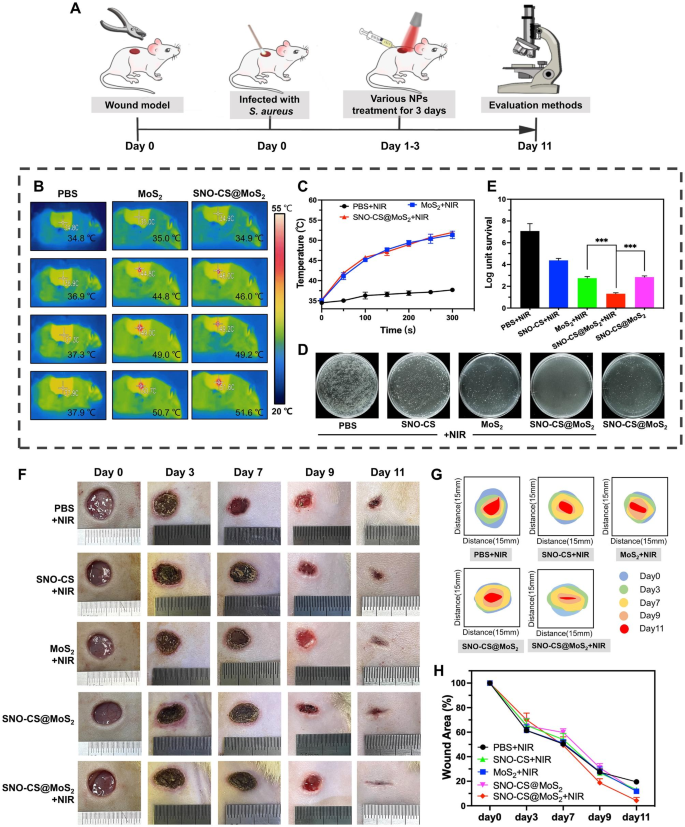
Impressed by the wonderful efficiency of SNO-CS@MoS2 in antibacterial, angiogenic, and speedy cell migration in vitro, a rat full-thickness pores and skin defect mannequin contaminated with S. aureus was developed to evaluate the efficacy of SNO-CS@MoS2 in contaminated wound therapeutic in vivo. As illustrated in Fig. 7A, the contaminated wounds had been handled with the supplies and the corresponding NIR irradiation for the primary 3 days of therapy. Determine 7B-C show the native adjustments in physique temperature on the contaminated wound website in the course of the first-day irradiation therapy. It may be noticed that SNO-CS@MoS2 exhibited glorious photothermal conversion efficiency in vivo. The temperature within the wound space elevated from 34.9 to 51.6 °C quickly after 5 min of irradiation. To find out the power to regulate NO era in vivo, The answer from the wound space of the rat’s again was collected and the focus of its NO was measured. The outcomes are confirmed that SNO-CS@MoS2 + NIR might launch practically 3.54 μmol L− 1 of NO, whereas SNO-CS@MoS2 with out NIR releases solely 0.53 μmol L− 1 of NO (Fig. S12). The in vivo launch of NO from SNO-CS@MoS2 was barely decrease than the above outcomes of experiments in vitro (Fig. 2H). A extra advanced setting within the physique might scale back the focus of NO launched attributable to wound exudate diluting the SNO-CS@MoS2 answer. As well as, behaviors equivalent to motion and licking can have an effect on the quantity of fabric retained within the wound, thus decreasing the quantity of NO launched. Though the focus of NO launched was decreased, it nonetheless met the efficient antibacterial focus. The in vivo antibacterial impact of SNO-CS@MoS2 is proven in Fig. 7D-E. The micro organism colony in wound tissue of SNO-CS@MoS2 + NIR group decreased from 6.58 to 1.52 log after the first-day therapy. Moreover, the synergistic antibacterial impact of PTT and large NO can additional be proved by evaluating with MoS2 + NIR and SNO-CS@MoS2 teams. This phenomenon can be supported by the anti-bacterial outcomes of drug-resistant micro organism in vivo (Fig. S13). These outcomes are in line with the in vitro antibacterial assay.
Bacterial an infection triggers an inflammatory response, together with the infiltration of inflammatory cells and the discharge of inflammatory mediators. These inflammatory responses not solely disrupt tissue construction but additionally inhibit cell proliferation and angiogenesis [57]. Killing micro organism successfully reduces the inflammatory response and promotes regular wound therapeutic [58]. To guage the therapeutic efficacy of nanomaterials in wound therapeutic, the wound therapeutic course of in every group was dynamically noticed. SNO-CS@MoS2 therapy considerably accelerated contaminated wound therapeutic, as verified by the outcomes illustrated in Fig. 7F-H. The wound measurement of SNO-CS@MoS2 + NIR group decreased to 2.95% on day 11, whereas within the different teams, it remained larger than 10%.
The effectivity of the SNO-CS@MoS2 on wound therapeutic. A) Schematic illustration for the institution of an contaminated wound mannequin and the following therapy regime. B–C) Thermal photos of rats below NIR irradiation and the corresponding photothermal heating curves. D–E) Cultured micro organism colonies on plates separated from wound tissues after therapy of day 1 and relative survival log of micro organism. F) The Digital pictures of wound closure in numerous therapy of PBS + NIR, SNO-CS + NIR, MoS2 + NIR, SNO-CS@MoS2 and SNO-CS@MoS2 + NIR on days 0, 3, 7, 9, and 11. G–H) Schematic diagram and quantification of the wound space after therapies on day 0, 3, 7, 9 and 11. (Laser: 808 nm, 1.0 W cm− 2, 10 min; Focus: [MoS2] = 200 μg mL− 1, [SNO-CS] = 30 μg mL− 1)
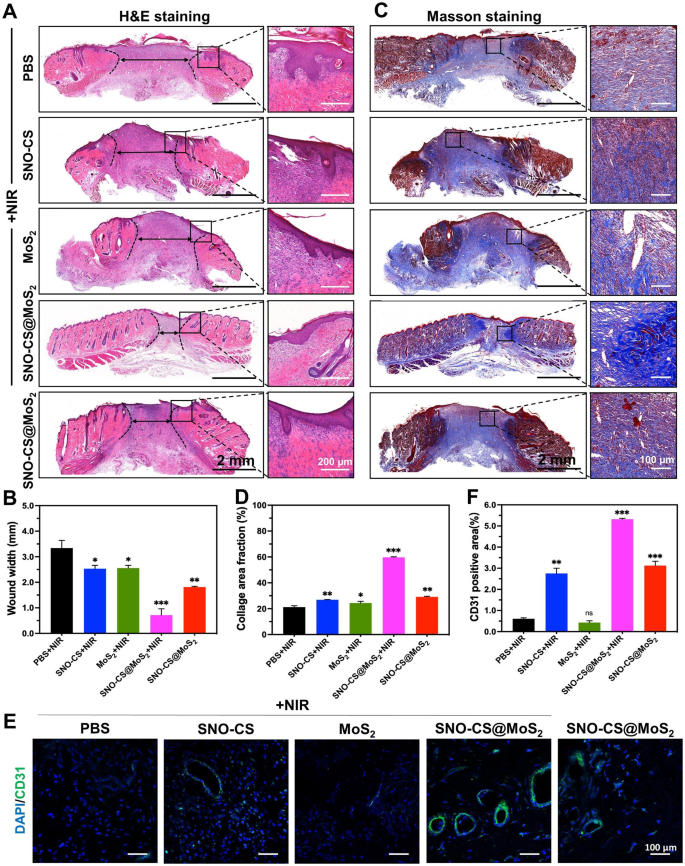
To additional conduct an intensive evaluation of the inflammatory reactions and the affect on blood vessel formation by SNO-CS@MoS2 in wound therapeutic, pores and skin wound tissues had been collected from rats on day 11 for histological experiments. Determine 8 A and C show that SNO-CS@MoS2 + NIR therapy decreased inflammatory cell infiltration within the wound tissue and considerably promoted wound re-epithelialization. In comparison with PBS and SNO-CS + NIR teams, the MoS2 + NIR and SNO-CS@MoS2 teams exhibited comparatively intact neo-keratin, however giant quantities of inflammatory cells had been nonetheless infiltrating. Notably, SNO-CS@MoS2 + NIR therapy group confirmed not solely a big discount in inflammatory cell infiltration, but additionally common epidermal constructions, well-proliferating fibroblasts, and even new child pores and skin attachments. Determine 8B and D show collagen deposition within the wound tissue after the completely different therapies. The SNO-CS@MoS2 + NIR group confirmed a better degree of collagen deposition in comparison with the opposite teams, with a brand new collagen space of roughly 60%. Moreover, the collagen fibers on this group had been denser and extra recurrently organized. These outcomes additional confirmed the power of hint NO to advertise the proliferation and migration of fibroblasts on the wound website. Moreover, NO regulates endothelial cell features equivalent to proliferation, migration, and tube formation, important for angiogenesis [59]. It can also modulate the expression of varied angiogenic elements, together with VEGF and fibroblast progress issue (FGF), influencing blood vessel formation [60]. CD31 was used for immunofluorescence staining to guage the angiogenesis-promoting impact of SNO-CS@MoS2. As anticipated, all NO-treated teams (SNO-CS + NIR, SNO-CS@MoS2, and SNO-CS@MoS2 + NIR) exhibited various levels of angiogenesis (Fig. 8E-F). Particularly, the SNO-CS@MoS2 + NIR group confirmed the best expression of CD31, ensuing from the gradual launch of NO, which significantly improve vascular regeneration.
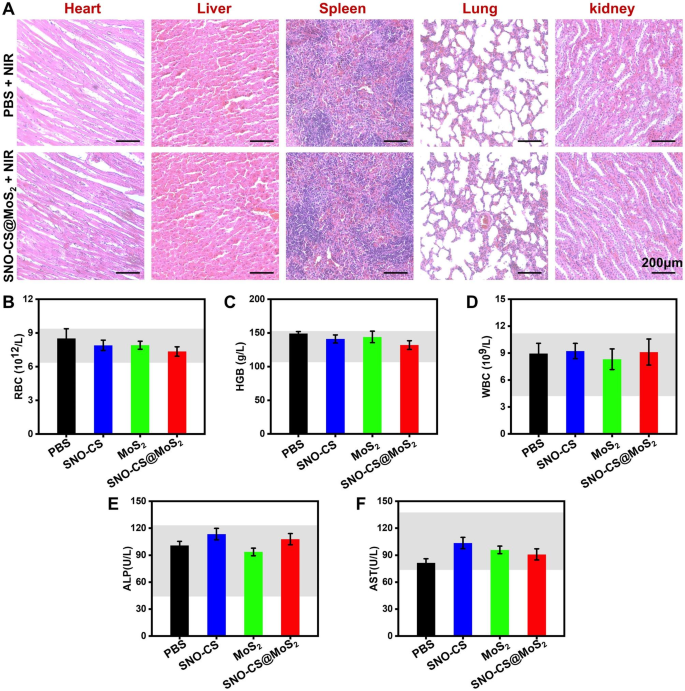
Contemplating the security of SNO-CS@MoS2 for scientific software, a complete in vivo evaluation of the biosafety was carried out. Histological analyses of main tissues, together with the center, liver, spleen, lung, and kidney, are illustrated in Fig. 9A, displaying no harm or irregular defects after SNO-CS@MoS2 + NIR therapy. Apart from, the values of the three consultant haematological indices involved (RBC, HGB and WBC) had been all throughout the regular vary (Fig. 9B-D). Indicators associated to liver perform (ALP and AST) had been additionally throughout the regular vary (Fig. 9E-F). Thus, the newly designed SNO-CS@MoS2 demonstrated glorious biocompatibility and biosafety, making them appropriate for scientific purposes. Taken collectively, these findings counsel that SNO-CS@MoS2 has glorious potential to advertise therapeutic of contaminated wounds, which could be attributed to the next elements: (1) antibacterial exercise and irritation management; (2) enhancement of native vascular regeneration; (3) promotion of wound fibrous deposition and epithelialization; (4) glorious biocompatibility. This progressive system holds immense potential in treating pores and skin infections primarily brought on by micro organism.
In vivo biocompatibility of SNO-CS@MoS2. (A) H&E staining of coronary heart, liver, spleen, lung, and kidney of PBS + NIR and SNO-CS@MoS2 + NIR teams. The routine blood evaluation for every therapy group on the eleventh day, (B) white blood cell rely (WBC), (C) hemoglobin (HGB), (D) purple blood cell rely (RBC), (E) alkaline phosphatase (ALP) and (F) aspartate aminotransferase (AST)