tPNPs sure a better variety of human than murine proteins
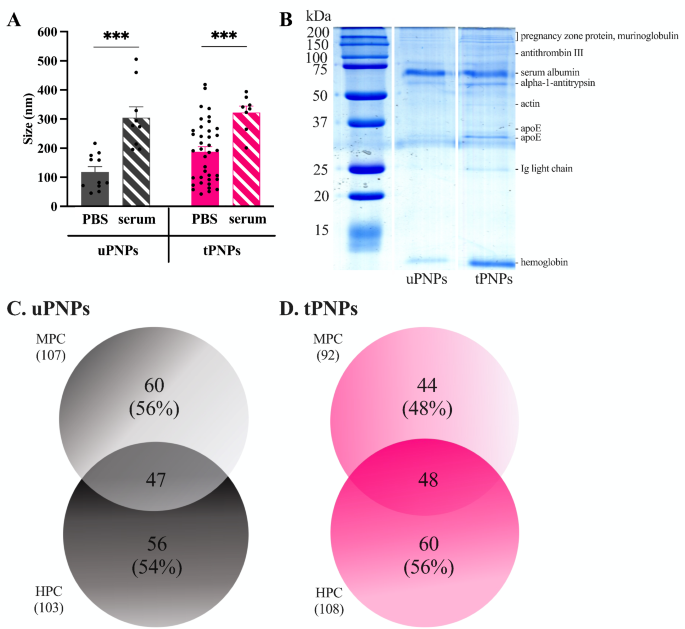
Two various kinds of PNPs have been produced for this examine: untargeted PNPs (uPNPs) and focused PNPs (tPNPs). Each nanostructures are fabricated from PEG, PCL, and PLA; the distinction between them is the conjugation of a human anti-CD20 antibody on the floor of the tPNPs, a concentrating on agent that’s absent on the uPNPs. To keep away from aggregation, PNPs have been saved in 0.3% BSA, that was primarily eliminated by means of centrifugation earlier than incubation with organic fluids. The PNPs have the same diameter (uPNPs vs. tPNPs 118 ± 61 nm vs. 189 ± 104 nm), taking in consideration the presence of antibodies on the floor, however after incubation with HS their measurement will increase considerably by ~ 150 nm (uPNPs in PBS vs. HS: 118 ± 61 nm vs. 305 ± 111 nm, p-value 0.0007. tPNPs in PBS vs. HS: 189 ± 104 nm vs. 323 ± 62 nm, p-value 0.0002; Fig. 1A).
Formation of mouse and human protein corona on PNPs. A. Detection of protein corona formation by TEM evaluation of PNPs earlier than and after incubation with serum. Information are represented as imply ± SEM and analyzed by the two-tailed Scholar’s t-test. Black spots signify single measurements. *** p-value <0.001. B. Deposition of proteins on PNPs was additionally detected by SDS-PAGE and Good Blue staining. The id of the bands was decided by LC-MS/MS evaluation and indicated on the fitting aspect of SDS-PAGE. The variety of totally different proteins detected on uPNPs (C) and tPNPs (D) was obtained by LC-MS/MS evaluation and reported in a Venn diagram. PBS: phosphate buffered saline; PNPs: polymeric nanoparticles; uPNPs: untargeted PNPs; tPNPs: focused PNPs; MPC: mouse protein corona; HPC: human protein corona
The rise of the dimension of each PNPs sorts after incubation with serum is because of each the formation of a PC surrounding the PNPs and the expansion of the PNPs themselves, as proven by the TEM evaluation (Further File 1: Supplementary Fig. 1A and 1B). This sample suggests a scarcity of stability of the nanocarriers in organic fluids, assuming a lack of perform of the PNPs in vitro and in vivo. Nevertheless, our previous research broadly demonstrated the security and efficacy of PNPs in mouse fashions of human cancers [21,22,23,24] and rheumatoid arthritis [25].
The formation of a PC surrounding the PNPs was confirmed by SDS-PAGE by which protein bands have been seen after Coomassie Good Blue staining, indicating the adsorption of serum proteins. PC was enriched in low molecular weight (MW) proteins (e.g., MW < 15 kDa, hemoglobin) and excessive MW (e.g., ~ 60 kDa, albumin), as urged by the presence of extra intense bands. Different seen bands signify Ig mild chains (25 kDa), apoE (33 kDa), actin (45 kDa), alpha-1-antitrypsin (52 kDa), antithrombin III (~ 100 kDa), and being pregnant zone proteins (> 200 kDa) (Fig. 1B). After incubation of the PNPs with HS or MS, the adsorbed proteins have been recognized by liquid chromatography-mass spectrometry (LC-MS/MS). Nearly the identical variety of proteins have been adsorbed to uPNPs; the truth is, 107 and 103 proteins have been detected within the murine protein corona (MPC) and the human protein corona (HPC), respectively. Amongst these, 56% of the murine proteins and 54% of the human proteins have been uniquely adsorbed to uPNPs (Fig. 1C).
The presence of the human antibody on the tPNPs prevented the binding of some murine proteins; the truth is, the MPC consisted of a smaller variety of proteins than the HPC (mouse vs. human proteins: 92 vs. 108). This sample could be defined by the species-specific interactions that an antibody can type with proteins within the serum of the identical mother or father organism. Subsequently, the bare polymer-forming uPNPs might work together indiscriminately with murine and human proteins, whereas the human antibody conjugated to the tPNPs might favor adsorption of some human proteins. Consequently, the proportion of distinctive proteins elevated, accounting for 48% of MPC in comparison with 56% of HPC (Fig. 1D).
Particles bind preferentially negatively charged and low-MW proteins
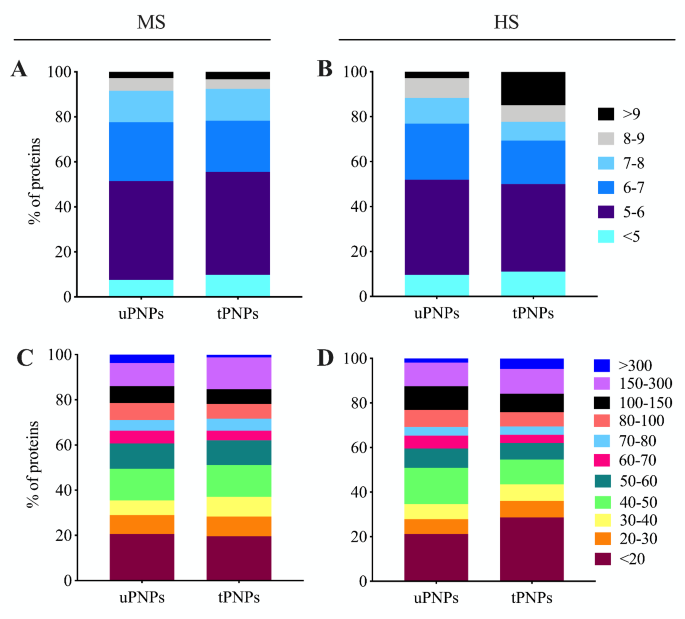
The classification of proteins adsorbed on PNPs and primarily based on MW and isoelectric level (IP) is a part of their bodily characterization. Each facets can be utilized to foretell the biochemical features of the detected molecules [26]. This evaluation additionally permits comparability between totally different species, resembling these thought of within the examine: Homo Sapiens and Mus Musculus. In each HPC and MPC, proteins with a unfavorable cost in serum predominate. Certainly, MPC of each PNPs was primarily represented by proteins with an IP between < 7, which accounted for 78% of the whole proteins detected (Fig. 2A). In distinction, when the PNPs have been incubated with HS, the variations have been evident. Negatively charged proteins (IP < 7) accounted for 77% of the HPC of the uPNPs, whereas this proportion decreased for the tPNPs (69%). One other vital distinction between particles is in human proteins with an IP > 9; such proteins, which carry a optimistic cost at physiological pH, have been detected 3 times extra ceaselessly on tPNPs, the place they accounted for as much as 15% of the HPC, than on the untargeted counterpart (3%, Fig. 2B).
Classification of adsorbed proteins primarily based on molecular weight and isoelectric level. Mouse and human proteins adsorbed to PNPs have been categorized primarily based on their molecular weight (A, B) and isoelectric level (C, D). The graphs have been plotted with the next proportion: whole variety of proteins detected : 100% = sum of proteins with the identical caharacteristics : x. MS: mouse serum; HS: human serum; uPNPs: untargeted nanoparticles; tPNPs: focused nanoparticles
IP evaluation represents the distribution of proteins primarily based on their cost and permits predictions concerning the attainable electrostatic interactions between PNPs and proteins. If serum proteins sure to PNPs solely by electrostatic interactions, the adsorption sample noticed could be simply predictable. As a result of unfavorable cost of the particles used on this examine [25], we had anticipated to detect a higher variety of positively-charged proteins within the PC. Nevertheless, it’s identified that the floor cost of PNPs impacts the conformational adjustments of proteins [27], which prevents their interplay with PNPs primarily based on their cost alone. Moreover, the PNPs used within the examine include hydrophobic polymers resembling PLA and PCL, and hydrophilic PEG. Hydrophobic polymers are identified to bind primarily negatively charged molecules [28]. In our settings, the PCs of each PNPs have been characterised by negatively charged proteins fairly than proteins carrying a optimistic cost.
PNPs have been additionally proven to preferentially adsorb small proteins. About 50% of the MPC consisted of proteins with MW < 50 kDa (uPNPs vs. tPNPs: 50% vs. 51%), of which 20% consisted of proteins smaller than 20 kDa. The one distinction between the particles is in proteins with excessive MW (MW between 150 kDa and 300 kDa), which account for 10% and 14% of the MPC of uPNPs and tPNPs, respectively. This sample is reverse taking in consideration proteins with MW above 300 kDa (uPNPs vs. tPNPs: 4% vs. 1%; Fig. 2C). When incubated with HS, about 50% of the proteins are these with MW beneath 50 kDa (uPNPs vs. tPNPs: 51% vs. 55%), as proven in mice. Amongst them, PNPs are primarily characterised by proteins with lower than 20 kDa; they accounted for 20% and 29% of the HPC of uPNPs and tPNPs, respectively. One other distinction between the particles is in human proteins between 40 and 50 kDa, which have been adsorbed extra by uPNPs than by tPNPs (uPNPs vs. tPNPs: 16% vs. 11%; Fig. 2D).
Human protein corona differentiated extra uPNPs from tPNPs in respect to the murine counterpart
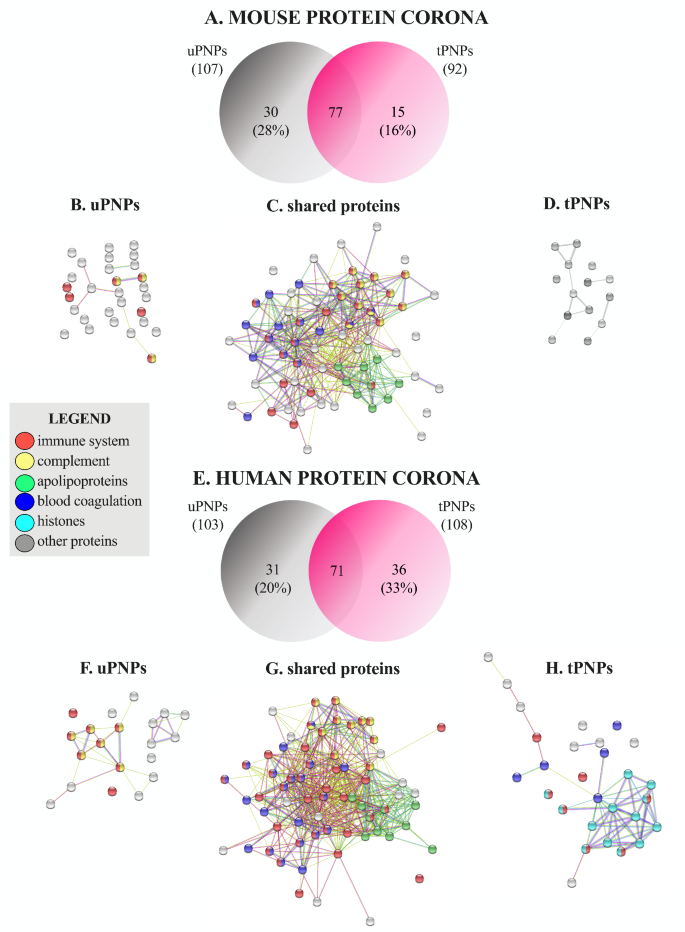
A extra detailed classification of MPC and HPC was carried out, contemplating singularly the sure proteins. The Venn diagram confirmed the presence of 77 frequent murine proteins together with further 30 distinctive proteins (28%) adsorbed to uPNPs, which is twice the proteins detected within the MPC of tPNPs (15 proteins, 16%, Fig. 3A).
Venn diagram and organic perform of adsorbed murine and human proteins. The proteins adsorbed to the PNPs have been detected by LC-MS/MS and categorized in keeping with their organic perform (e.g., immune system involvement, complement activation, blood coagulation, apolipoproteins, histones, and others). The quantity and proportion of mouse proteins are proven in a Venn diagram (A). Proteins adsorbed solely on uPNPs (B), these shared by the particles (C) and people current solely on tPNPs (D) are proven by string evaluation. The identical was for human proteins: Venn diagram (E), proteins adsorbed to uPNPs (F) or shared by each particles (G) or current solely on tPNPs (H). uPNPs: untargeted nanoparticles; tPNPs: focused nanoparticles
The distinction between PNPs is because of some proteins concerned in C activation (Fig. 3B), together with C1r and C1s, subcomponents of C1, and C9 (Further File 2: Supplementary Desk 1), and concerned in immune response (Fig. 3B), resembling antileukoproteinase, Beta-2-microglobulin and histidine-rich glycoprotein (Further File 2: Supplementary Desk 1). Along with these proteins, apolipoproteins (Apos) are of nice significance in a PC, as they play a major position in blood circulation, in PNPs capability to cross organic obstacles [26] and in C activation as effectively. As for the late course of, Apo A-I and Apo A-II inhibit C9 polymerization and hinder the formation of the membrane assault advanced (MAC) [29]. An analogous position is performed by Apo J (also referred to as clusterin), which, in synergy with vitronectin, inactivates the terminal complexes C5b-9 [30]. All detected Apos (Apo A-I, Apo A-II, Apo A-IV, Apo B-100, Apo C-I, Apo C-III, Apo C-IV, Apo E and Apo M; Further File 2: Supplementary Desk 1) have been shared by each particle sorts (Fig. 3C); Apo B-100 and Apo A-IV signify the 4th and the fifth most plentiful proteins of the MPC of uPNPs (Further File 2: Supplementary Desk 1). The tPNPs didn’t adsorb distinctive proteins concerned within the immune system, C activation or coagulation processes (Fig. 3D). This sample was partially in step with that discovered after incubating the particles with HS. Certainly, 72 frequent proteins have been shared by each PNPs, and 31 (30%) and 36 (33%) further distinctive proteins have been detected on the floor of uPNPs and tPNPs, respectively (Fig. 3E). The variations between PNPs have been primarily attributable to proteins concerned in C activation, the coagulation course of, and histones. A barely greater variety of human proteins concerned within the C-activation pathway, resembling C4, C6, C8 (beta and gamma chains), issue B and issue H (Further File 3: Supplementary Desk 2), have been detected solely on the floor of uPNPs (Fig. 3F). Alternatively, the HPC of tPNPs was extra enriched in proteins concerned within the coagulation course of, together with coagulation issue X and XIII-A, integrin α-Ib and ß-3, and multimerin-1 (Further File 3: Supplementary Desk 2).
As for human Apos, Apo A-I, Apo A-II, Apo A-IV, Apo B-100, Apo C-I, Apo C-II, Apo C-III, Apo C-IV, Apo D and Apo E (Further File 3: Supplementary Desk 2) have been current on each PNPs (Fig. 3G); abundances have been additionally comparable (information not proven). Apo B-100 and Apo E signify the first and 4th most plentiful proteins of the HPC of each particles. Apo A-I is the fifth most plentiful protein adsorbed on tPNPs (Further File 3: Supplementary Desk 2). Surprisingly, a better variety of histones, primarily H2B, was detected within the HPC of tPNPs (Fig. 3H). The binding of histones to PNPs is nothing new within the literature. Certainly, negatively charged PEGylated liposomes [31] and colloidal gold PNPs [32] have been proven to bind core histones resembling H2A, H2B, H4 and H2B, H4, respectively. Free histones are typically launched after necrosis of dying cells and are a part of neutrophil extracellular traps (NETs); in our context, their involvement in immune activation and restore processes [33] might affect the religion of PNPs after injection. Nevertheless, their perform continues to be unknown.
Murine serum fails to obviously distinguish uPNPs from tPNPs
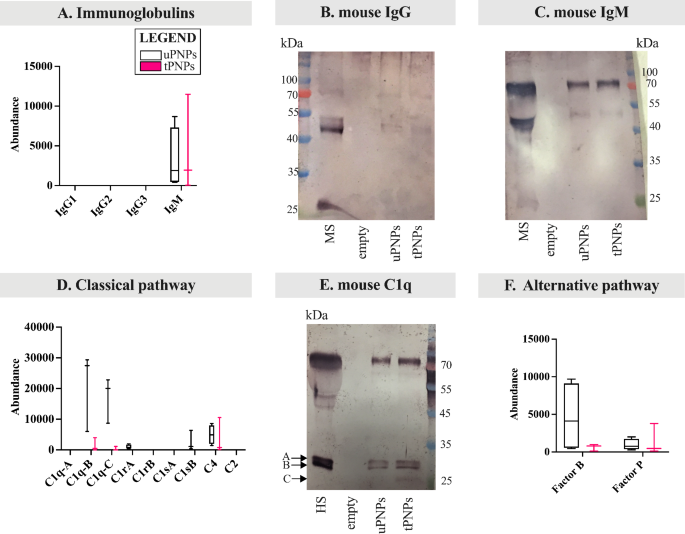
With extra consideration to C deposition and/or activation, we’ve targeted on the totally different parts of the cascade, together with immunoglobulins (Igs). Concerning the latter, murine IgG (e.g., IgG2 and IgG3) and IgM isotypes are identified to strongly activate murine C [34]. Amongst them, IgG weren’t adsorbed to PNPs, whereas IgM have been extra current within the MPC of tPNPs in comparison with uPNPs (Fig. 4A). These information, obtained by LC-MS/MS evaluation, have been additionally confirmed by WB. The IgG band (mouse IgG Fc (fragment crystallizable) area: 50 kDa) was not seen (Fig. 4B), whereas the fixed area of IgM was detected on each particles with the attended measurement of 75 kDa (Fig. 4C).
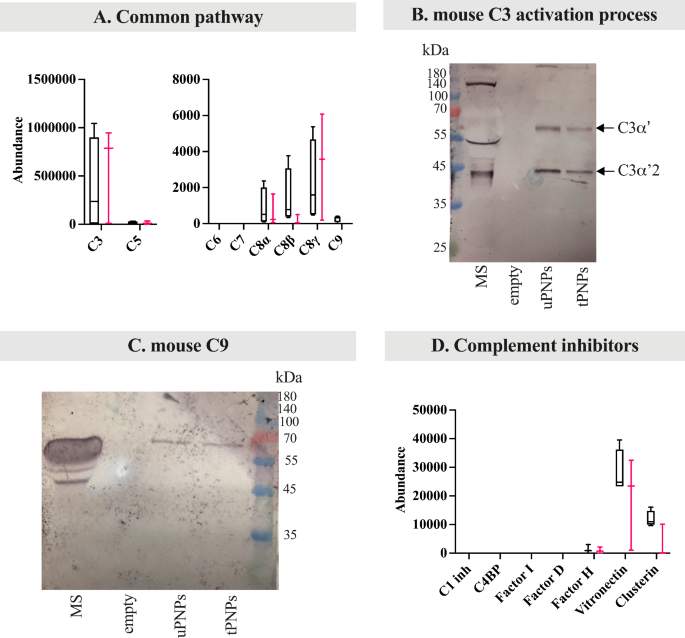
Deposition of parts of the mouse complement system on PNPs. The proteins adsorbed on PNPs have been analyzed by LC-MS/MS and Western blot (WB) analyses. Immunoglobulins have been quantified by LC-MS/MS (A) and the deposition of mouse IgG (B) and IgM (C) was additionally analyzed by WB (attended band at 50 kDa and 75 kDa, respectively). Elements of the classical complement activation pathway have been analyzed by LC-MS/MS evaluation (D); the corresponding bands of C1q chains have been visualized by WB (E, attended bands top: ~26 and 27 kDa). Proteins of the choice pathway (F) deposited on particles have been detected by LC-MS/MS evaluation. LC-MS/MS information (n=4) are introduced as abundance values (arbitrary values generated by mass spec peak integration) and are proven as boxplot with median worth. uPNPs: untargeted nanoparticles; tPNPs: focused nanoparticles; MS: mouse serum; empty: empty effectively
IgG and IgM are accountable for activating the classical pathway of C after the binding to the C1 advanced [35], which in flip consists of three subcomponents important for its exercise: C1q, shaped from three chains (A, B and C), C1r and C1s [36]. After activation, C1 cleaves C4 and C2 into bigger (C4b, C2b) and smaller (C4a, C2a) fragments. The catalytic exercise of C1q results in the formation of the C4bC2b advanced, often called “C3 convertase”, which subsequently cleaves C3 into the anaphylatoxin C3a and the opsonin C3b [37]. Absorbed parts of the classical C activation pathway by each PNPs embrace the B and C chains of C1q, C1r, C1s and C4. Apart from C4, the opposite proteins have been extra plentiful on uPNPs, which was additionally the one sort of nanostructure that sure C1rA and C1sB. The latter proteins are one of many two isoforms ensuing from gene duplication in mice that results in the formation of C1rA, C1rB and C1sA, C1sB [38]. C1rB and C1sA weren’t detected (Fig. 4D). The deposition of C1q was additionally confirmed by WB evaluation; the 2 bands of murine C1q (A sequence (UNIPROT P98086 – C1QA_MOUSE) and C chain (UNIPROT Q02105 – C1QC_MOUSE): 25.974 kDa and 25.992 kDa, respectively, and B chain (UNIPROT P14106 – C1QB_MOUSE): 26.717 kDa) have been seen on each particles (Fig. 4E). The band at ~ 75 kDa is the results of aspecific binding of the secondary antibody recognizing additionally IgM-Fc portion.
To additional distinguish between C deposition and activation, the merchandise of C4 cleavage have been analyzed individually. C4a, a potent anaphylatoxin, is launched from the C4 molecule to stimulate irritation, whereas C4b stays on the cell floor to recruit different C parts and proceed the activation cascade [37]. Thus, when C is activated, C4a shouldn’t be detected. To evaluate the presence of this fragment, LC-MS/MS peptides have been mapped to the unique sequence of C4a, indicating that it’s, a minimum of partially, adsorbed on each particles and never a particular binding consequence of C1 activation (Further File 4: Supplementary Fig. 2A).
Different pathway is initiated by the spontaneous auto-activation of C3 (C3H2O) into two molecules: C3a and C3b. C3b binds the goal after which Issue B, cleaved by Issue D, to type C3bBb; this advanced is additional stabilized by Issue P [9]. In our settings, Issue B and Issue P have been detected on each particles, however with reverse binding patterns: Issue B was extra strongly adsorbed by uPNPs, whereas tPNPs confirmed greater abundance of Issue P (Fig. 4F).
Proteins particularly concerned within the lectin pathway weren’t detected (information not proven).
C3 acts as a convergence level of the three C pathways and was proven to be current on each PNPs, on the identical abundance (Fig. 5A).
Deposition of mouse frequent complement parts and inhibitors of the complement system on PNPs. The proteins adsorbed on PNPs have been analyzed by LC-MS/MS (information introduced as abundance values, arbitrary values generated by mass spec peak integration, n = 4) and Western blot (WB) analyses. LC-MS/MS information are proven as boxplot with median worth. The deposition of frequent complement proteins (e.g., C3, C5, C6, C7, C8, C9) was quantified by LC-MS/MS analyses (A). To higher consider complement activation, C3 fragmentation was studied by WB (B, C3α’: 63 kDa; C3α’2: 40 kDa). WB was additionally used to spotlight the deposition of C9 (C, attended band at ~ 75 kDa), a terminal element of the complement activation. Complement inhibitors (D) have been quantified by LC-MS/MS evaluation. uPNPs: untargeted nanoparticles; tPNPs: focused nanoparticles; MS: mouse serum; C1 inh: C1 inhibitor; C4BP: C4 binding proteins; empty: empty effectively
As well as, the detection of C3a, often launched from the C3 molecule after C activation [37], could present a sign of its standing. Peptides mapped within the C3a area have been current on each uPNPs and tPNPs, suggesting solely a partial C3 activation (Further File 4: Supplementary Fig. 2B), as beforehand described for C4. This speculation was supported by the presence of C3 activation bands within the WB evaluation. Along with the lack of C3a (9 kDa) from the C3α chain of C3, there’s additionally its fragmentation into smaller components: C3α’ (63 kDa), C3α’2 (40 kDa) and C3f (2 kDa). C3α’ is cleaved once more into two components: considered one of 23 kDa and C3dg (40 kDa); the C3β chain stays intact (75 kDa) [39] (Further File 4: Supplementary Fig. 2C). The WB evaluation confirmed solely two different seen bands at ~ 60 kDa and ~ 40 kDa, representing C3α’ and C3α’2, respectively. The band comparable to the small fragment of C3α’ (23 kDa) was not detected (Fig. 5B).
The classical and different pathways converge to the identical last steps: one other C3 molecule binds to the beforehand shaped C3 convertase to type C5 convertases (C4bC2bC3b and C3bBbC3b as C5 convertases of the classical different pathway, respectively), which in flip cleaves C5 to generate C5a and C5b. Particularly, C5a is launched from the molecule, whereas C5b types the MAC by means of the affiliation with C6, C7, C8 and C9 and lyses cells [37]. Wanting extra carefully on the late pathway, solely C5 and C8 (α, ß and γ chains) have been detected on the floor of each PNPs, whereas C9 was solely evidenced on uPNPs. As with C4a and C3a, peptides mapping to the sequence of C5a have been additionally analyzed: C5a was not current to the PNPs (Further File 4: Supplementary Fig. 2D), indicating the whole activation of sure C5. C6 and C7 weren’t detected (Fig. 5A). The WB evaluation additionally confirmed the presence of C9 to uPNPs; nonetheless, the attended band at ~ 75 kDa [40] was additionally seen on tPNPs (Fig. 5C).
To keep away from constitutive activation of C in inappropriate contexts, there are a number of inhibitory proteins that act at totally different levels of the pathway. Briefly, the classical C activation pathway is inhibited by C1 inhibitor and C4BP. C1 inhibitor binds to the energetic website of C1r and C1s and inhibits the activated C1 advanced [41], whereas C4BP controls the meeting of C3 convertase. C4BP can also be concerned within the regulation of the lectin pathway which causes the cleavage and inactivation of C4b. By its a number of binding websites for C3b, Issue H additionally regulates the choice C pathway and blocks the binding of issue B to type C3 convertase. Different inhibitory proteins that have an effect on all three pathways are Issue I, Issue D, vitronectin and clusterin. Particularly, Issue I, together with cofactors resembling Issue H and C4BP, cleaves C3b and C4b [42]. Issue D counteracts the formation of C3 convertase; particularly, for the choice pathway, Issue D cleaves Issue B when it’s sure to C3b [43]. Lastly, clusterin and vitronectin affiliate with terminal complexes (C5b-9, C5b-7, C5b-8) to stop their insertion into lipid bilayers of membranes [44]. After incubation with MS, solely Issue H, clusterin, and vitronectin have been adsorbed in virtually equal quantities on each PNPs, whereas C1 inhibitor, C4BP, Issue D, and Issue I weren’t detected (Fig. 5D). All detected C inhibitors lastly intervene with the deposition of the late parts and the formation of MAC, in order that their detection on each PNPs signifies that murine C activation could be prevented by this mechanism. Apart from C1 advanced parts, which have been extra adsorbed on uPNPs, there are little variations between particles suggesting that mouse serum is unable to distinguish focused from untargeted nanostructures in vitro.
The antibody conjugated to tPNPs mediates the preferential binding of proteins of the human classical pathway, whereas the polymers preferentially bind molecules of the human different pathway
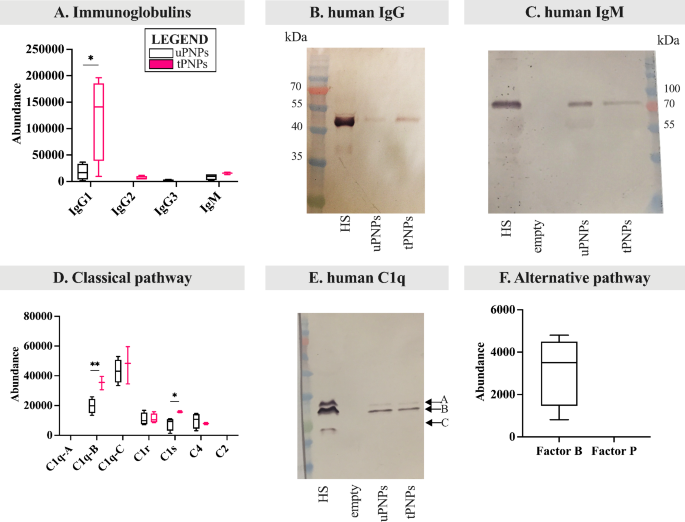
The identical strategy used for MPC, to evaluate the deposition of C system proteins, was additionally used for HPC. As for Igs, IgG1, IgG2, IgG3 and IgM are identified to strongly activate the human C system [45, 46]. Subsequently, our consideration was initially targeted on the adsorption of those molecules. tPNPs have been proven to current higher quantities of IgG1 (uPNPs vs. tPNPs: 17,895 ± 15,421 vs. 121,895 ± 80,129, p-value 0.029) and low quantity of IgG2, which was absent on uPNPs, and IgM; then again, uPNPs bind low quantity IgG3, which is absent within the HPC of tPNPs (Fig. 6A).
Deposition of proteins of the human complement system on PNPs. The adsorbed proteins have been analyzed by LC-MS/MS and Western blot (WB) analyses. A. Adsorbed immunoglobulins resembling IgG (i.e., IgG1, IgG2, IgG3) and IgM have been detected by LC-MS/MS. The deposition of human IgG (B) and IgM (C) was additionally analyzed by WB evaluation (attended band: 50 kDa and 75 kDa, respectively). Elements of the classical complement activation pathway have been analyzed by LC-MS/MS evaluation (D) and the bands comparable to C1q chains have been visualized by WB (E, attended bands of C1q at ~ 23, 27 and 29 kDa). Proteins of the choice (F) pathway have been detected by LC-MS/MS evaluation. LC-MS/MS information are proven as boxplots with median worth. * p-value < 0.05; ** p-value < 0.01. uPNPs: untargeted nanoparticles; tPNPs: focused nanoparticles; HS: human serum; HP: human plasma; empty: empty effectively
IgG adsorption was confirmed by WB evaluation (human IgG Fc portion, 50 kDa; Fig. 6B). The very excessive abundance of human IgG on tPNPs with respect to uPNPs could be defined by the presence of the human-Fc antibody conjugated to the floor, which doesn’t essentially point out a particular binding sample of IgG to tPNPs however a management of the strategy we have been utilizing. As for IgM, the band comparable to the Fc portion (75 kDa, Fig. 6C) was extra intense when uPNPs have been thought of, which is in contradiction with the LC-MS/MS information (Fig. 6A).
The classical C activation pathway is mediated by the C1 advanced, as described for mice. The upper abundance of IgG1 and IgM detected on tPNPs corresponds to elevated deposition of the C1 advanced. Regardless that chain A of C1q was not detected and chain C was current on each particles with out vital distinction, chain B of C1q was considerably extra documented on tPNPs than on uPNPs (uPNPs vs. tPNPs: 19,773 ± 5064 vs. 35,266 ± 4529, p-value 0.009). The identical sample was noticed for C1s, which was statistically extra plentiful on tPNPs than on their untargeted counterpart (uPNPs vs. tPNPs: 7766 ± 4390 vs. 15,806 ± 630, p-value 0.033). Alternatively, C1r was adsorbed onto each particles in the same abundance (Fig. 6D). The deposition of C1q was additionally investigated by WB evaluation. The presence of the three seen bands by means of which C1q is shaped (C1q chain A, B and C: 29, 27 and 23 kDa, respectively [47]) confirmed its adsorption on each PNPs (Fig. 6E). To evaluate the activation of C through the classical pathway, C2 and C4 molecules have been additionally thought of. C2 was undetected, whereas C4, together with C4a (Further File 5: Supplementary Fig. 3A), was adsorbed by each particles (Fig. 6D). These information urged partial or lack of C activation.
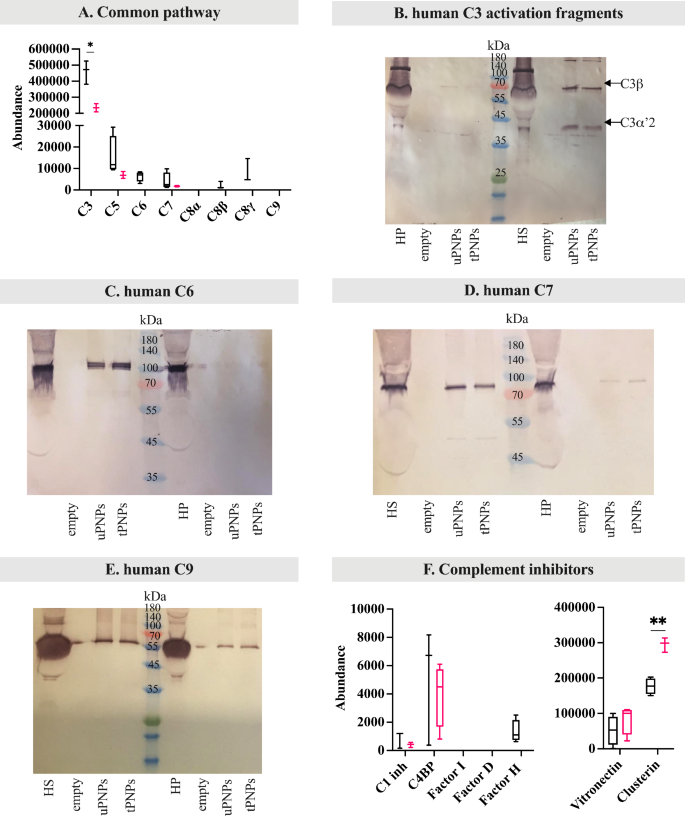
The choice pathway of C activation in people follows the identical steps beforehand described for mice. In our settings, Issue B was documented solely on uPNPs, whereas it was absent on tPNPs. Issue P was undetected in any respect (Fig. 6F). Together with Issue B, C3 additionally confirmed considerably greater abundance on uPNPs in comparison with tPNPs (uPNPs vs. tPNPs: 459,079 ± 72,796 vs. 234,834 ± 22,377, p-value 0.001; Fig. 7A), confirming the correlation between these two molecules through the activation of the choice pathway; certainly, spontaneous auto-activation of soluble C3 or response of C3b with hydroxyl or amino teams of PNPs is thought to mediate the formation of C3bBb convertase.
Deposition of human frequent pathway complement proteins and inhibitors on PNPs. The adsorbed proteins have been analyzed by LC-MS/MS and Western blot (WB) analyses. The deposition of proteins of the frequent pathway of complement activation (C3, C5, C6, C7, C8 and C9) was quantified by LC-MS/MS (A). To higher consider complement activation, C3 fragmentation was analyzed by WB (B, C3ß: 63 kDa; C3α’2: 40 kDa). WB was additionally used to visualise the deposition of C6 (C, attended band at ~ 100 kDa), C7 (D, attended band at ~ 95 kDa) and C9 (E, attended band at ~ 70 kDa). Complement inhibitors have been quantified by LC-MS/MS evaluation (F). uPNPs: untargeted nanoparticles; tPNPs: focused nanoparticles; HS: human serum; HP: human plasma; empty: empty effectively; C1 inh: C1 inhibitor; C4BP: C4 binding protein
This, in flip, results in an enhancement of C3b opsonization and thus activation of the choice pathway [9].
The variations between untargeted and focused PNPs are evident: tPNPs preferentially adsorbed parts of the classical C activation pathway in accordance with the presence of the human-Fc antibody, whereas uPNPs have been extra liable to adsorb proteins of the choice pathway.
To evaluate C activation, mapping of LC-MS/MS peptides to C3a and WB evaluation of C3 fragments have been carried out. C3a was current on each NP programs, once more indicating partial or absence of C activation (Further File 5: Supplementary Fig. 3B). This outcome was according to the WB evaluation; after incubation with HS, two bands have been seen: one at ~ 75 kDa and one at ~ 40 kDa comparable to C3β and C3α’2 (or C3dg), respectively. The absence of different bands, resembling these at 63 kDa (C3α) and 23 kDa (C3α with out C3dg fragment), in addition to the absence of serious bands after the incubation with HP (the place the activation of the C system was blocked by the presence of EDTA), suggests C activation a minimum of as much as C3 stage (Fig. 7B).
The parts of the late pathway, particularly C5, C6 and C8 (ß and γ chains), have been predominantly adsorbed on uPNPs. Quite the opposite, C7 was current within the HPC of each particles, whereas C9 was undetected (Fig. 7A). As already studied for C3 and in mouse, peptides comparable to the sequence of C5a have been detected solely within the HPC of uPNPs, whereas they have been absent on tPNPs (Further File 5: Supplementary Fig. 3C). The absence of C5a on the HPC of tPNPs urged the whole activation by the C5 convertases; in all probability, only a quote of C3 and C4 have been hydrolyzed, as a result of C3a and C4a detection on tPNPs.
An extra WB evaluation was carried out to evaluate the binding of C6, C7 and C9, evaluating the incubation of the PNPs with HS or HP. C6 and C7 have been detected on each particles within the presence of HS; certainly, the attended bands have been seen at ~ 100 and ~ 95 kDa [48], respectively. With plasma, then again, C6 and C7 weren’t detected, suggesting an activation a minimum of as much as C7 stage (Fig. 7C and D, respectively). A discrepancy arose when C9 was thought of. In reality, C9 was not current after LC-MS/MS evaluation, whereas the attended band at ~ 70 kDa [48] was seen in WB after incubation of the PNPs with HS but in addition with HP, suggesting a binding unbiased from C activation (Fig. 7E).
As for inhibitors, C1 inhibitor, C4, vitronectin and clusterin have been detected on each PNPs, whereas Issue H was adsorbed solely on the floor of the uPNPs. Amongst them, clusterin confirmed considerably elevated abundance on tPNPs (uPNPs vs. tPNPs: 176,928 ± 22,377 vs. 295,173 ± 20,315, p-value 0.001; Fig. 7F). The presence of other and late pathway parts and the absence of inhibitors on uPNPs suggests attainable activation of C from the start to the tip by the choice pathway. In distinction, the absence of late pathway proteins and the presence of a considerably greater quantity of inhibitors adsorbed on tPNPs indicated much less C activation on these particles.
tPNPs activated extra C by means of classical and lectin pathways
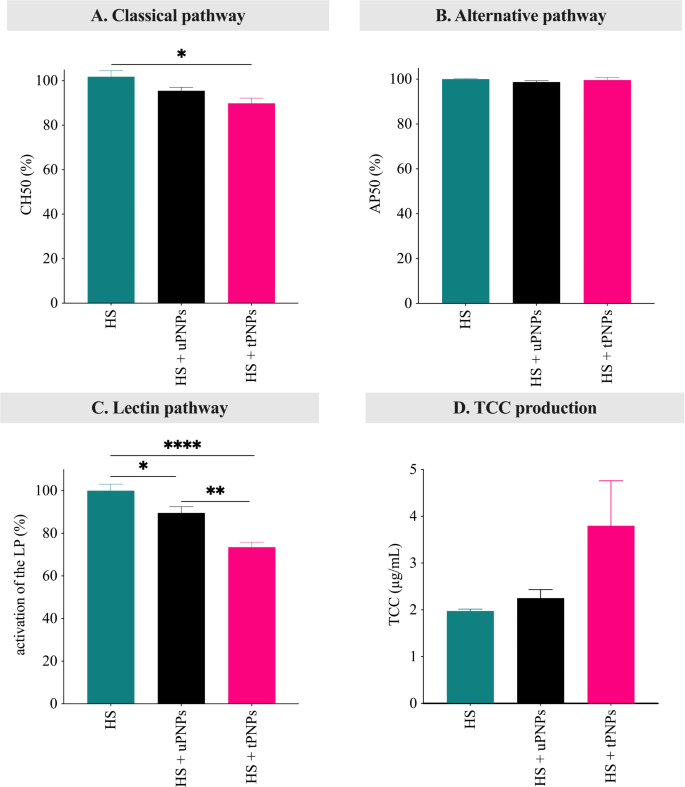
LC-MS/MS evaluation, along with WB, gives indications of C activation standing. To totally elucidate the contribution of the concentrating on mechanism conjugated to the PNPs in C activation, hemolytic assays and ELISA have been carried out to guage residual C exercise and C-activation merchandise in human serum. Hemolytic assays have historically been primarily based on the easy photometric measurement of hemoglobin launch, following lysis of RBCs mediated by C activation. This technique can selectively exploit classical or different C activation pathways utilizing Ab-sensitized sheep RBCs [49] or rabbit RBCs [50]. Though mouse usually mimics human processes, HS stays the popular pattern for C activation research in vitro. Certainly, it has been demonstrated that in vitro hemolytic exercise of mouse C is roughly 200- to 300-fold decrease than human C [51]. PNPs have been incubated with HS and, after centrifugation, the supernatant containing the residual C exercise was incubated with sheep or rabbit RBCs. Untreated HS was used as a optimistic management, retaining the whole quantity of C exercise. Per the upper deposition of C1 advanced parts on tPNPs, they have been proven to barely cut back the residual exercise of the classical pathway (Fig. 8A; HS vs. tPNPs-treated HS: 100 ± 4.2 vs. 89.9 ± 4; p-value 0.034).
Activation of the human complement by PNPs. Classical (A) and different (B) pathway activation was evaluated by lytic assays. The activation of lectin pathway (C) and the deposition of TCC (D) have been assessed by ELISA. Information are proven as imply ± SEM, n = 3. Information have been analyzed by one-way ANOVA. * p-value < 0.05; ** p-value < 0.01; *** p-value < 0.001; **** p-value < 0.0001. HS: human serum; uPNPs: untargeted nanoparticles; tPNPs: focused nanoparticles; CH50: 50% of hemolytic exercise induced by the classical pathway; AP50: 50% of hemolytic exercise induced by the choice pathway; LP: lectin pathway; abs: absorbance; TCC: terminal complement advanced
Two additional discrepancies have been discovered when inspecting activation of the choice and lectin pathway. Though Issue B, C3 and the late pathway proteins adsorbed extra to uPNPs, no vital activation of the choice pathway was detected for any of the nanostructures (Fig. 8B). Moreover, no deposition of lectin pathway proteins was detected by LC-MS/MS. Surprisingly, the lectin pathway was essentially the most diminished among the many three pathways. In reality, each PNPs have been discovered to considerably activate the lectin pathway (HS vs. uPNPs-treated HS: 100 ± 7.4 vs. 89.6 ± 7.1, p-value 0.044. HS vs. tPNPs-treated HS: 100 ± 7.4 vs. 73.5 ± 5.6, p-value < 0.0001), with tPNPs inflicting considerably greater C consumption in comparison with uPNPs (uPNPs vs. tPNPs: 89.6 ± 7.1 vs. 73.5 ± 5.6, p-value 0.0025. Fig. 8C). This may be attributable to a discount of the focus of different parts shared with the totally different C pathway; particularly, C4 and C2 with the classical pathway and C3 and the late parts with each classical and different pathway.
To verify C activation, the manufacturing of the TCC was evaluated detecting C5b-C9 advanced by ELISA. Each particles confirmed TCC manufacturing after incubation with HS however with out vital distinction between particles (Fig. 8D).
The activation of C on PNPs impacts macrophages engulfment however is just not influenced by concentrating on abs
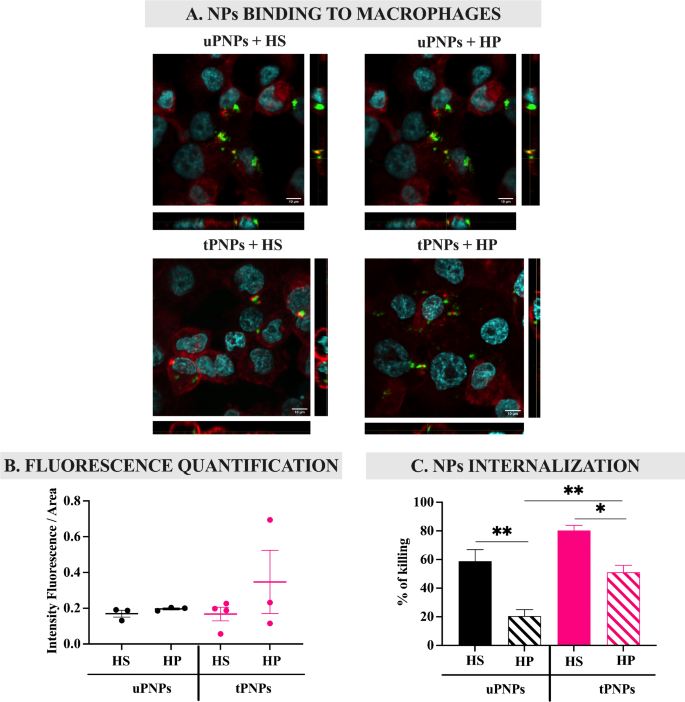
The liver is the primary website of elimination of PNPs after injection [23]. This course of is mediated by the massive variety of macrophages on this organ, which, in flip, specific on their floor particular receptors for overseas constructions [7] which were opsonized by molecules, together with C activated merchandise. Subsequently, the contribution of C within the interplay of nanostructures with macrophages was assessed in vitro by evaluating PNPs incubated with HS (inflicting C deposition) and HP (by which the C system is blocked). In short, fluorescent PNPs have been incubated with HS or HP and introduced in touch with human macrophages (PMA-stimulated THP-1 cells); the binding of the PNPs to the cells was analyzed by confocal microscopy (Fig. 9A).
PNPs binding and internalization inside macrophages after complement activation. PMA-stimulated THP-1 cells have been incubated with HS- or HP-PNPs for 1 h. Confocal microscopy photographs have been acquired (A) and the quantity of fluorescence was quantified with Fiji software program (B). To evaluate PNPs internalization inside cells, drugs-loaded PNPs have been incubated for 1 h with human macrophages. Unbound PNPs have been faraway from cells and the proportion of dying cells was quantified by MTT assay (C). Information are represented as imply ± SEM; n = 3. Statistical evaluation was carried out utilizing a one-way ANOVA take a look at. * p-value < 0.05; ** p-value < 0.01. uPNPs: untargeted nanoparticles; tPNPs: focused nanoparticles; HS: human serum; HP: human plasma
Macrophages have been discovered to work together with PNPs independently of C activation; certainly, incubation of PNPs with HS didn’t improve binding to the cells in contrast with HP (Fig. 9B). Nevertheless, the distinction between the particles turned clear evaluating PNPs internalization, which was assessed by quantifying the proportion of macrophages killed after publicity to drugs-loaded PNPs. First, tPNPs with out C activation (tPNPs + HP) have been internalized considerably greater than the untargeted counterpart (uPNPs vs. tPNPs: 20.4 ± 9.1% vs. 51.2 ± 9.6%, p-value 0.004). This sample might be as a result of binding of the human Fc portion of the antibody conjugated to tPNPs to particular receptors expressed on macrophages, together with THP-1 cells [52]. After incubation with HS, the internalization of each PNPs within the cells elevated considerably in comparison with HP (uPNPs HP vs. HS: 20.4 ± 9.1% vs. 58.8 ± 13.9%, p-value 0.021. tPNPs HP vs. HS: 51.2 ± 9.6% vs. 80.3 ± 6.2%, p-value 0.005), confirming the hyperlink between C activation and macrophage engulfment, however no variations have been noticed evaluating focused and untargeted particles incubated with HS (Fig. 9C); these information show the same opsonization of the nanostructure, not ample to extend macrophage engulfment, unbiased from the presence of the concentrating on antibody and inducing the same last elimination by phagocytosis after PC formation.