Physics is crammed with mysteries. To seek out just a few value exploring, look no additional than an ice dice. At room temperature, in fact, the dice will soften earlier than your eyes. However even far beneath freezing, ice can shift in just perceptible ways in which scientists are nonetheless attempting to know. Utilizing imaging instruments on the U.S. Division of Vitality’s (DOE) Argonne Nationwide Laboratory, researchers have detected a phenomenon generally known as premelting at temperatures far decrease than these beforehand noticed.
Their findings are printed within the journal Proceedings of the Nationwide Academy of Sciences.
Premelting is the explanation {that a} patch of ice might be slippery even on a frigid, clear day. Although the spot is frozen, some half on the floor is moist, an thought first posited by Michael Faraday within the mid-1800s. The thought of a premelted, liquid-like layer on ice opens up different longstanding questions on how water transforms from liquid to strong to vapor—and the way, underneath sure situations, it may be all three directly.
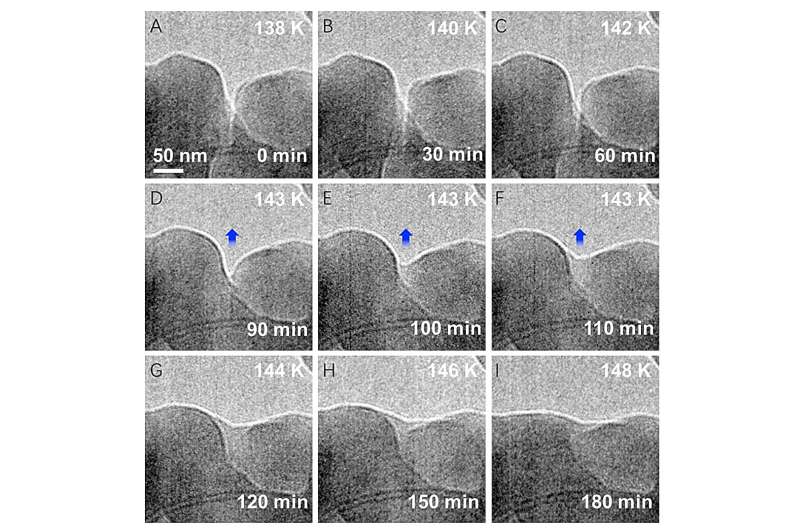
Within the current research, scientists examined ice crystals fashioned beneath minus 200 levels Fahrenheit. The staff used Argonne’s Heart for Nanoscale Supplies (CNM), a DOE Workplace of Science person facility, to develop and observe the ice nanocrystals, which measured solely 10 millionths of a meter throughout.
Apart from what the research reveals concerning the nature of water at subfreezing temperatures, it demonstrates a way for analyzing delicate samples in molecular element: low-dose, high-resolution transmission electron microscopy (TEM). TEM directs a stream of electrons, that are subatomic particles, at an object. A detector creates a picture by selecting up how the electrons scatter off the thing.
“Some supplies are beam-sensitive. If you use an electron beam to picture them, they are often modified or destroyed,” mentioned Jianguo Wen, Argonne supplies scientist and a lead creator on the paper. One instance of an electron beam delicate materials is electrolytes, which alternate charged particles in batteries.” Having the ability to research them in nice element with out disrupting their construction might assist in the event of higher batteries.
However to begin, researchers are experimenting with the low-dose TEM method on frozen water. In any case, water is affordable and plentiful. Greater than that, Wen mentioned, “Ice could be very difficult to picture, as a result of it’s so unstable underneath the high-energy electron beam. If we efficiently reveal this method on ice, imaging different beam-sensitive supplies might be a chunk of cake.”
The low-dose method combines the CNM’s aberration-corrected TEM with a specialised direct electron detection digicam. The system is extraordinarily environment friendly at capturing info from every electron that hits a pattern, so it’s potential to get a high-resolution picture utilizing fewer electrons, thus inflicting much less harm on the goal than a traditional TEM method.
The low degree of electron publicity makes it potential to seize one thing as delicate as an ice crystal in situ, or in its atmosphere. The analysis staff used liquid nitrogen to develop the ice crystals on carbon nanotubes at 130 levels Kelvin, or minus 226 levels Fahrenheit.
Earlier research had noticed premelting near water’s triple level. On the triple level, the temperature is only a hair above freezing and the strain is low sufficient that ice, liquid and water vapor can exist directly. At temperatures and pressures beneath triple level, ice sublimates straight into water vapor.
The “guidelines” of water’s habits are sometimes neatly summed up in a easy section diagram that maps out water’s various states throughout totally different mixtures of temperature and strain.
“However the true world is way more complicated than this straightforward section diagram,” mentioned Tao Zhou, Argonne supplies scientist and one other corresponding creator of the paper. “We confirmed that premelting can occur far down on the curve, although we can not clarify why.”
In a video captured in the course of the experiment, two separate nanocrystals might be seen dissolving into one another because the ice is warmed underneath fixed strain to 150 levels Kelvin, or minus 190 levels Fahrenheit. Although nonetheless nicely beneath freezing, the ice fashioned a quasi-liquid-like layer. This ultraviscous water will not be accounted for among the many easy traces of the section diagram, the place water goes straight from ice to vapor.
The research raises intriguing questions that may very well be explored in future work. What’s the actual nature of the liquid-like layer the researchers noticed? What would occur if the strain is raised, together with the temperature? And does this method pave the way in which towards a glimpse of “no-man’s land,” the state the place super-cooled water all of a sudden crystallizes from liquid into ice? The centuries-long scientific inquiry into water’s many states continues.
Co-authors with Wen and Zhou are Lei Yu, Thomas Gage, Suvo Banik, Arnab Neogi, Henry Chan, Xiao-Min Lin, Martin Holt, and Ilke Arslan of Argonne; Yulin Lin and Aiwen Lei of Wuhan College; and Nathan Rosenmann of the College of Illinois at Chicago.
Extra info:
Yulin Lin et al, Floor premelting of ice far beneath the triple level, Proceedings of the Nationwide Academy of Sciences (2023). DOI: 10.1073/pnas.2304148120
Supplied by
Argonne Nationwide Laboratory
Quotation:
Even far beneath freezing, ice’s floor begins melting as temperatures rise (2024, January 4)
retrieved 4 January 2024
from https://phys.org/information/2024-01-ice-surface-temperatures.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for info functions solely.


