There’s an ever-present battle to cut back carbon-based power sources and exchange them with low or no-carbon options. The method of splitting water might be the decision.
Hydrogen manufacturing is an easy, secure, and efficient methodology to provide extra power than gasoline can by the easy means of splitting water. Harvesting power this fashion, versus relying closely (or in any respect) on carbon-based power sources, is more and more changing into the usual. Researchers have discovered a technique to make use of transition steel sulfides, like tin (Sn), cobalt (Co), and iron (Fe) on nickel foam, to develop non-precious steel electrocatalysts to be used in cost-effective and environmentally accountable water splitting.
The researchers have printed their ends in Nano Analysis Power.
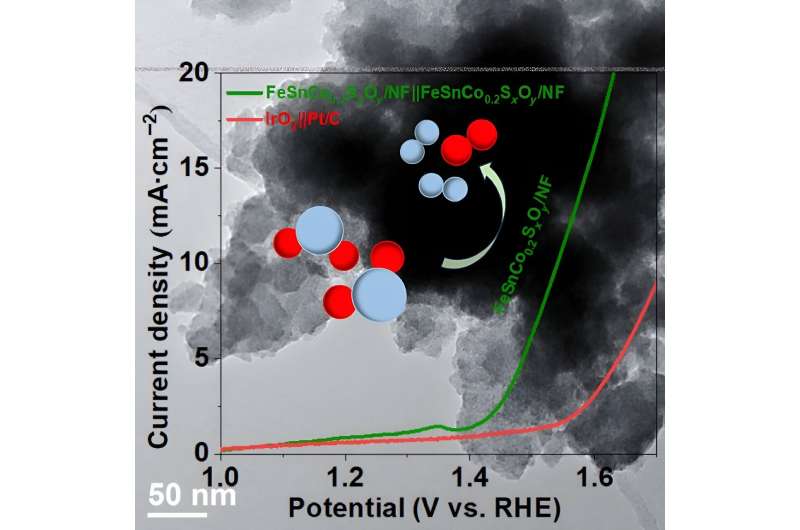
To achieve success on this carbon-reducing enterprise, some reactions have to be stabilized for this course of. The star of the research is FeSnCo0.2SxOy/NF, which might act as each an anode and cathode within the means of splitting water at a low voltage.
The 2 reactions of concern listed below are oxygen evolution reactions (OER) and hydrogen evolution reactions (HER). OER generates O2 by way of a chemical response from water. HER yields H2 from a two-electron switch response. The ensuing H2 is beneficial as gasoline. Utilizing each of those reactions is good for making a bifunctional electrocatalyst. Electrocatalysts might be outlined as catalysts (or reaction-starters) that operate at electrode surfaces, that are surfaces that may carry {an electrical} present.
HER has proved to be steady at 55 hours of steady use and in addition requires a decrease overpotential than OER. Overpotential is the distinction within the quantity of power wanted for a given catalyst to function.
Sadly, OER stability isn’t the place it must be. That is partially as a result of additional step concerned within the electron switch but in addition as a result of the electrolytes they operate below are usually harsh. Whereas OER is steady with steady use of round 70 hours, its exercise does lower with extra cobalt content material.
“It’s pivotal to enhance the OER stability of transition steel sulfides in order that they can be utilized as bifunctional HER and OER catalysts for reversible hydrogen gasoline cells,” stated Jingqi Guan, creator and researcher of the research.
OER additionally has a better overpotential than HER. With a better quantity of power wanted to induce the catalyst into operation, OER might be extra “troublesome.” Nonetheless, the mix of iron, tin, and cobalt on nickel foam boasts some enchancment in bifunctional stability and each HER and OER exercise.
The mix of those metals and the heterostructural interfaces shaped can regulate the distribution of the electrons throughout the electrolyte floor. “Heterostructural” right here refers to a semiconductor that may have an altered chemical composition based mostly on the place the 2 chemical substances are in. On this occasion, it’s a sulfide/oxyhydroxide duo.
Even distribution of electrons helps to extend the speed of cost switch all through the entire construction, which then promotes the switch of electrons. As a result of nature of this semiconductor, rising stability naturally would enhance total exercise and performance.
General, these transition metals have a synergistic impact on one another, particularly when present process HER. This impact makes them superb candidates for the primary problem proposed by researchers: decreasing carbon-based power sources.
Although the outcomes had been very promising, there are all the time steps that may be made sooner or later to good a course of. Discovering a catalyst that minimizes the overpotentials can scale back the power enter wanted to catalyze the response. Moreover, making certain the electrocatalysts developed are sturdy sufficient for use commercially and may stand up to lengthy hours of steady utilization with none in poor health results is crucial to the long-term success of the heterostructural interfaces.
Extra info:
Siyu Chen et al, Interface engineering of Fe-Sn-Co sulfide/oxyhydroxide heterostructural electrocatalyst for synergistic water splitting, Nano Analysis Power (2023). DOI: 10.26599/NRE.2023.9120106
Offered by
Tsinghua College Press
Quotation:
Engineering non-precious steel electrocatalysts for cost-effective and environmentally accountable water splitting (2023, November 30)
retrieved 1 December 2023
from https://phys.org/information/2023-11-non-precious-metal-electrocatalysts-cost-effective-environmentally.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for info functions solely.


