Though ammonia (NH3) is seen to be a viable carbon-free power supply, specialists all through the world are nonetheless puzzled by its energy-intensive manufacturing methodology. To provide ammonia from nitrate (NO3-), a analysis crew led by the Metropolis College of Hong Kong (CityU) just lately engineered a bimetallic alloy as an ultrathin nanocatalyst that may present considerably enhanced electrochemical efficiency. This gives nice potential for the long run manufacturing of carbon-neutral gas.
The outcomes, titled “Atomic coordination setting engineering of bimetallic alloy nanostructures for environment friendly ammonia electrosynthesis from nitrate,” had been printed within the Proceedings of the Nationwide Academy of Sciences (PNAS) journal.
Since ammonia is simpler to liquefy and transport than hydrogen, and may function a supply of hydrogen for gas cells, this often-used fertilizer ingredient has garnered numerous curiosity these days. Upcycling nitrate (NO3-) from wastewater contaminated by ammonium fertilizers has grow to be a viable different for creating helpful ammonia and enhancing the sustainability of agriculture attributable to its excessive demand.
As of proper now, the electrochemical nitrate discount response (NO3RR) is considered a viable methodology for producing ammonia. Metallic-based electrocatalysts principally encompass deoxygenation and hydrogenation phases (NO3– + 9H+ + 8e– ➙ NH3 + 3H2O).
Nonetheless, the undesired by-products and the competing hydrogen evolution response (HER) throughout NO3RR apparently hinders the yield charge of ammonia manufacturing.
Zhanxi Fan, Research Lead and Professor, Division of Chemistry, Metropolis College of Hong Kong
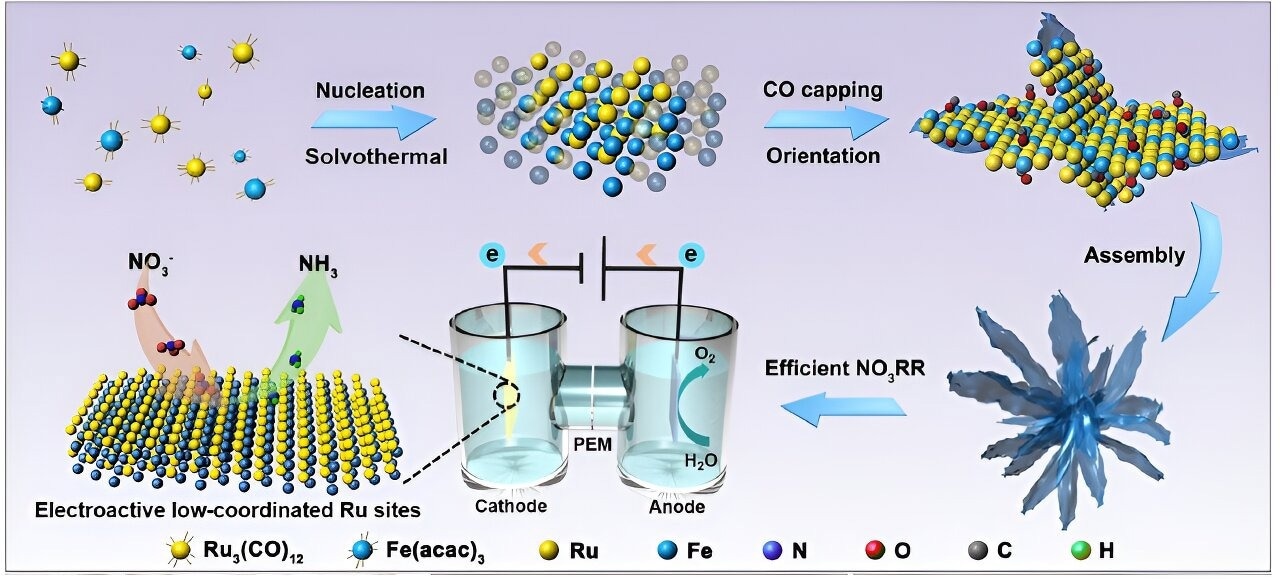
Not like prior research that altered the dimensions or dimensions of the electrocatalysts, Professor Fan’s group targeting enhancing the lively websites, that are the factors on the electrocatalyst floor the place substrate molecules connect and catalysis happens.
Fan added, “Ruthenium (Ru) is an rising materials as an electrocatalyst for NO3RR, nevertheless it additionally has the issue of favoring HER, which results in its lively websites being extremely occupied by undesired lively hydrogen, leaving inadequate space for nitrate discount into ammonia.”
Iron (Fe) was added by the researchers to change the atomic coordination setting of the lively websites to get across the difficulties. The digital buildings and floor traits of Ru, and due to this fact their catalytic exercise for ammonia manufacturing, are adjusted by modifying the coordination setting of the Ru websites.
The group created RuFe nanoflowers, ultrathin nanosheets organized right into a flower-like form, utilizing a one-pot synthesis methodology to additional enhance the electrocatalyst’s effectivity.
Along with suppressing the aggressive HER and decreasing the power limitations for NO3RR, the complementary orbitals on this distinctive bimetallic alloy-made catalyst obtain robust valence states and a extremely steady digital construction.
As well as, the RuFe nanoflowers’ electrochemically lively floor websites measured 267.5 cm2, which is much greater than the Ru-nanosheets’ 105 cm2 required for the reactions to happen.
The electrochemical efficiency of RuFe nanoflowers was remarkably improved, as evidenced by their distinctive faradaic effectivity (FE) of 92.9% and yield charge of 38.68 mg h−1 mgcat−1 for ammonia manufacturing at −0.30 and −0.65 V. This yield charge is sort of 6.9 occasions greater than that of sole Ru-nanosheets.
Fan concluded, “This analysis signifies nice potential for RuFe nanoflowers in next-generation electrochemical power programs. We consider this work can stimulate follow-up research on modulating the atomic coordination setting of lively websites in metal-based catalysts for ammonia manufacturing, additional selling a sustainable nitrogen cycle to realize carbon-free power sooner or later.”
Journal Reference:
Wang, Y, et. al. (2023) Atomic coordination setting engineering of bimetallic alloy nanostructures for environment friendly ammonia electrosynthesis from nitrate. Proceedings of the Nationwide Academy of Sciences. doi:10.1073/pnas.2306461120
Supply: https://www.cityu.edu.hk/


