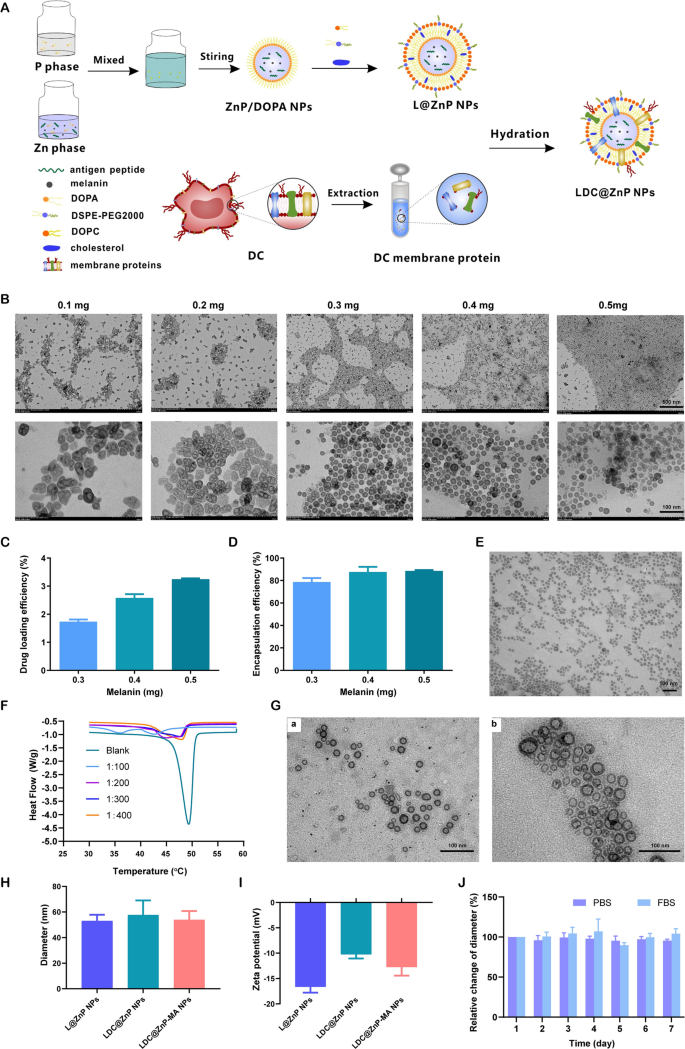
Preparation and characterization of LDC@ZnP NPs
The peptide and melanin co-loaded LDC@ZnP NPs had been fabricated by reverse microemulsion mixed with film-hydration methodology [22]. The schematic illustration of preparation was proven in Fig. 1A. The NPs formation primarily consisted of two steps: (1) preparation of separate Zn and P part underneath the existence of DOPA in cyclohexane/Igepal CO-520 system. (2) formation of zinc phosphate precursors [26]. As proven in Extra file 1: Fig. S1, clean ZnP NPs displayed common morphology and homogeneous distribution. To find out whether or not melanin impacted NP formation, completely different quantity of melanin (0.1 mg, 0.2 mg, 0.3 mg, 0.4 mg and 0.5 mg) was added into the oil system of Zn part, respectively. After interplay with P part, the nanoparticles had been collected by centrifugation, dispersed with chloroform and noticed by TEM. As proven in Fig. 1B, when the quantity of melanin was 0.3–0.5 mg, the nanoparticles offered often near-spherical with the optimum morphology at 0.4 mg. The loading effectivity of melanin by the nanoparticles was additional decided. The drug loading effectivity elevated with the rising enter of melanin (Fig. 1C). For the encapsulation effectivity, when the quantity of melanin was 0.4 and 0.5 mg, each the encapsulation fee was round 90% (Fig. 1D). Because the ready ZnP NPs had been hydrophobic, the NPs required hydrophilic modification for additional software. Lipid envelopment is a generally used methodology to change the floor traits of nanoparticles to enhance the steadiness and biocompatibility [27]. To acquire hydrophilic NPs, the ZnP NPs in chloroform had been spun along with DOPC, ldl cholesterol, and DSPE-PEG2000 (molar ratio 4:4:1) to kind a skinny movie. After hydration, lipid-enveloped zinc phosphate nanoparticles (L@ZnP NPs) had been obtained, which displayed comparable morphology as ZnP NPs (Fig. 1E). The hydrophobic nanoparticles had been remodeled into nanoparticles that might disperse stably in aqueous answer by lipid envelopment, which solved the aggregation downside of ZnP NPs and was extra conducive to the appliance of nanoparticles underneath physiological circumstances.
To additional functionalize L@ZnP NPs with dendritic cells, dendritic cell membrane proteins with completely different protein/phospholipid weight ratios (1:0, 1:100, 1:200, 1:300, 1:400) had been added into PBS for hydration. Adopted by an intense vortex for 3 min, ultrasound for 3 min, and incubation at 37 oC water bathtub for 30 min, dendritic cell membrane protein hybrid zinc phosphate nanoparticles (LDC@ZnP NPs) had been obtained. The protein profile of LDC@ZnP NPs was decided by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). As proven in Extra file 1: Fig. S2, the protein in LDC@ZnP NPs matched with the father or mother cells, indicating the profitable incorporation of DC membrane proteins in LDC@ZnP NPs. And the key membrane protein elements may contain integral or lipid-anchored plasma membrane, cytosolic, cytoskeletal, peripheral, and secreted proteins [28, 29]. Then, the nanoparticles with completely different quantities of dendritic cell membrane proteins had been freeze-dried and weighed. The corresponding temperature rise curve was obtained by a differential scanning calorimeter (DSC). As proven in Fig. 1F, when the protein/phospholipid weight ratio was 1:300 and 1:400, the nanosystem fashioned a single peak, indicating that cell membrane proteins had been uniformly inserted into the phospholipid layer on the floor of the nanoparticles. For a bigger quantity of dendritic cell membrane protein insertion, 1:300 was chosen to arrange the dendritic cell membrane protein hybrid nanovaccine. TEM was additional used to look at the membrane protein hybrid nanoparticles. As proven in Fig. 1G (a), the insertion of membrane proteins didn’t have an effect on the morphology of melanin-loaded hybrid nanoparticles (LDC@ZnP-M NPs). Then the antigen peptide Adpgk and melanin co-loaded dendritic cell hybrid nanoparticles (LDC@ZnP-MA NPs) had been ready by the same methodology. When the drug-fed quantities had been 0.4 mg melanin and 0.25 mg peptide, the encapsulation effectivity of melanin and peptide by nanovaccine had been 87.6 ± 4.6% and 57.2 ± 10.0%, respectively. The morphology of drug co-loaded hybrid nanoparticles confirmed a virtually spherical distribution as displayed in Fig. 1G (b).
Then the hydration particle dimension and zeta potential of nanoparticles had been measured by DLS. As proven in Fig. 1H, I, in contrast with L@ZnP NPs, the particle dimension and potential values of clean LDC@ZnP NPs and drug-coloaded LDC@ZnP-MA NPs had been barely elevated. The particle dimension was round 30 nm whereas the zeta potential was about − 10 mV. The steadiness of nanoparticles in PBS and FBS was additional monitored by DLS, and there was no vital distinction within the change of particle dimension throughout one week (Fig. 1J).
Preparation and characterization of LDC@ZnP NPs. A Schematic illustration for preparation of LDC@ZnP NPs. B TEM picture of ZnP NPs with completely different quantities of melanin including. The dimensions bar for the higher layer was 500 nm. The dimensions bar for the decrease layer was 100 nm. C Drug loading effectivity by ZnP NPs with completely different quantities of melanin including (n = 3). D Encapsulation effectivity by ZnP NPs with completely different quantities of melanin including (n = 3). E TEM picture of L@ZnP-M NPs, scale bar = 100 nm. F DSC curves of LDC@ZnP NPs with completely different quantities of DC membrane protein insertion. G TEM photographs of LDC@ZnP-M NPs (a) and LDC@ZnP-MA NPs (b), scale bar = 100 nm. H Hydration particle dimension of L@ZnP NPs, LDC@ZnP NPs and LDC@ZnP-MA NPs (n = 3). I Zeta potential of L@ZnP NPs, LDC@ZnP NPs and LDC@ZnP-MA NPs (n = 3). J Stability of LDC@ZnP NPs in PBS and FBS (n = 3)
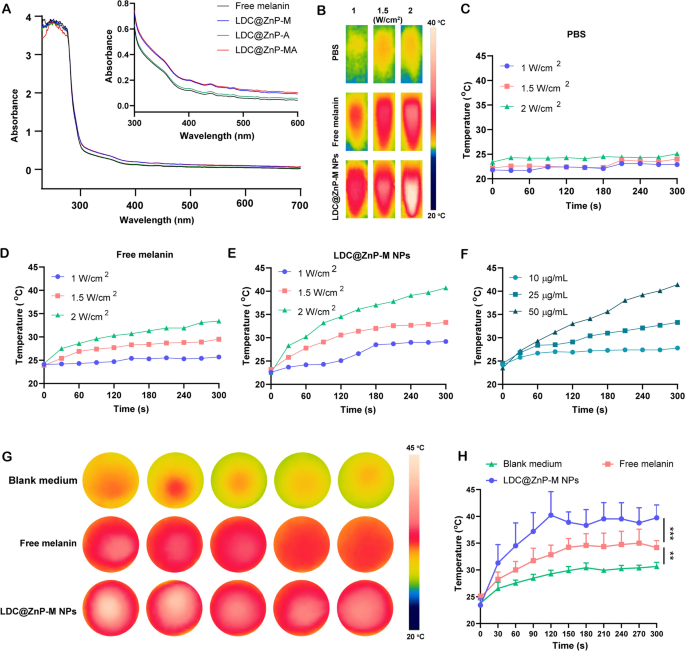
The in vitro photothermal impact of LDC@ZnP-M NPs
Firstly, UV absorption of free melanin, Adpgk loaded dendritic cell hybrid nanoparticles (LDC@ZnP-A NPs), LDC@ZnP-M NPs, and LDC@ZnP-MA NPs was detected by UV-VIS absorption spectroscopy, respectively. Free melanin is poorly soluble in PBS whereas LDC@ZnP-M NPs and LDC@ZnP-MA NPs enhanced the soluble standing of melanin by nanoparticle entrapment. And the improved solubility is correlated with the absorption in UV spectra [30]. Because of this, LDC@ZnP-M NPs and LDC@ZnP-MA NPs confirmed equally stronger absorption within the close to infrared area than free melanin and LDC@ZnP-A NPs (Fig. 2A). Then to observe the photothermal impact underneath NIR irradiation, PBS, Free melanin and equal LDC@ZnP-M NPs had been uncovered to 808 nm laser at completely different laser powers. The actual-time temperature of every group was recorded by infrared thermal imaging digital camera. As proven in Fig. 2B–E, PBS confirmed negligible temperature change underneath completely different energy. Free melanin and LDC@ZnP-M NPs confirmed enhanced photothermal impact with the rise of irradiation energy and time. Notably, LDC@ZnP-M NPs displayed extra outstanding temperature enhance than free melanin. Among the many three irradiated powers, 2 W/cm2 irradiation may obtain a glad mild-heat impact of LDC@ZnP-M NPs and was thereby chosen for follow-up experiments. Then the nanoparticles had been diluted right into a collection of concentrations with PBS and irradiated with 2 W/cm2 laser. As proven in Fig. 2F, the photothermal impact of LDC@ZnP-M NPs was in a dose-dependent method. Furthermore, the photothermal impact of LDC@ZnP-MA NPs (50 µg/mL melanin, 20 µg/mL Adpgk) was monitored underneath 2 W/cm2 irradiation for five min. A proven in Extra file 1: Fig. S3, LDC@ZnP-MA NPs displayed comparable profile with LDC@ZnP-M NPs, indicating that the peptide loading confirmed negligible influence on photothermal impact of NPs. Then the intracellular photothermal impact of LDC@ZnP-M NPs was investigated. BMDCs had been handled with clean medium, free melanin (50 µg/mL) and equal LDC@ZnP-M NPs, respectively. After 4 h, the supernatant was discarded and PBS was added. The plate was positioned at room temperature and irradiated with 808 nm laser at 2 W/cm2. Temperatures had been recorded at completely different time factors (0 s, 30 s, 60 s, 90 s, 120 s, 150 s, 180 s, 210 s, 240 s, 270 s, 300 s). As Fig. 2G, H confirmed, in contrast with clean medium and free melanin, LDC@ZnP-M NPs elevated quickly inside 120 s after which maintained at about 42 ℃, displaying a very good intracellular delicate photothermal impact.
Photothermal impact of LDC@ZnP NPs. A UV absorption spectra of free melanin and completely different nanoparticles. B Photothermal imaging of PBS, free melanin (50 µg/mL) and equal LDC@ZnP-M NPs underneath NIR irradiation for five min with completely different irradiation powers. C–E Photothermal profiles of PBS (C), free melanin (D) and LDC@ZnP-M NPs (E). F Photothermal profiles of LDC@ZnP-M NPs with completely different concentrations. G Photothermal photographs of clean medium, free melanin and LDC@ZnP-M NPs (n = 5). H Photothermal profiles of clean medium, free melanin and LDC@ZnP-M NPs (n = 5), **p < 0.01, ***p < 0.001
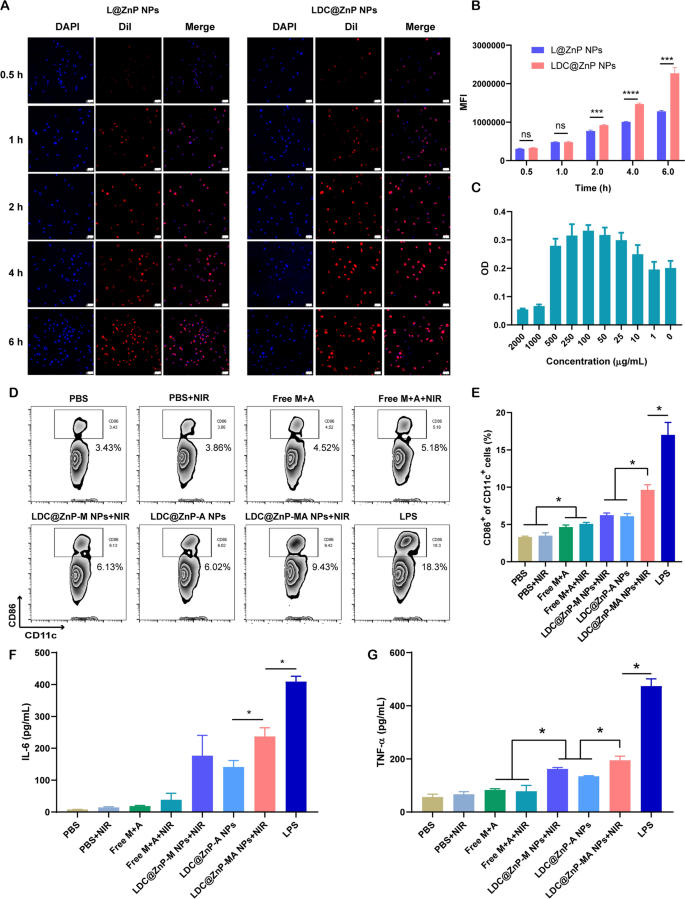
The in vitro interplay of LDC@ZnP NPs and BMDCs
L@ZnP NPs and LDC@ZnP NPs had been labeled with fluorescent dye DiI [31], and incubated with BMDCs for 0.5, 1, 2, 4, and 6 h, respectively, to find out the internalization behaviors of NPs. To visualise the intracellular uptake, the cells had been stained with DAPI and noticed by confocal microscope. As proven in Fig. 3A, the uptake of L@ZnP and LDC@ZnP NPs by DCs was time-dependent. Inside a comparatively brief interval of 0.5 and 1 h, homologous focusing on of LDC@ZnP NPs to DCs was not apparent. With incubation time prolonging, the uptake of LDC@ZnP NPs by DCs was remarkably enhanced than that of L@ZnP NPs. Moreover, stream cytometry was used for quantitative evaluation of cell uptake. It confirmed an analogous tendency because the outcomes of confocal photographs (Fig. 3B). These outcomes indicated that the hybrid of DC membrane proteins to L@ZnP NPs may obtain the homologous focusing on impact to DCs. It might be attributed to the molecule-mediated homotypic cell floor interactions, which might promote intracellular antigen accumulation in DCs [32].
After uptake antigen, immature DCs must endure maturation for antigen presentation [33]. Right here, the cytotoxicity of LDC@ZnP-MA NPs to BMDCs was firstly investigated earlier than conducting different assays. LDC@ZnP-MA NPs had been diluted right into a collection of concentrations (NPs quantity) with medium. As displayed in Fig. 3C, LDC@ZnP-MA NPs induced toxicities to BMDCs at excessive doses of 1000 and 2000 µg/mL. When the dose was decrease than 500 µg/mL, the NPs had been secure for DCs. Notably, DCs had been proliferative on the doses various from 10 to 500 µg/mL in comparison with the management group, suggesting that LDC@ZnP-MA NPs promoted the proliferation of BMDCs. It might be attributed to that the stimulation to DC promotes the enlargement of DCs [34, 35]. Then the photothermal security was investigated when mixed with NIR irradiation. 5 µg/mL and 10 µg/mL of LDC@ZnP-M NPs (akin to 193 and 385 µg/mL NPs) had been used to incubate with BMDCs. After co-incubating for 4 h, the supernatant was discarded and changed with contemporary medium. Then the cells had been positioned on the thermostat managed scorching plate (37 ℃) to take care of the cell viability, irradiated with 808 nm laser for various time and continued to tradition for one more 20 h. After irradiating for two min, the temperature of 5 µg /mL and 10 µg /mL LDC@ZnP-M NPs (melanin quantity) handled cells had been 38.9 ± 0.6 ℃ and 41.2 ± 0.8 ℃, respectively, which had been not more than the cytotoxic temperature above 42 ℃. As proven in Extra file 1: Fig. S4, each 5 µg /mL and 10 µg /mL LDC@ZnP-M NPs confirmed good photothermal security in comparison with clean medium. Notably, the cell viabilities of LDC@ZnP-M NPs handled cells had been remarkably larger than clean medium, which might be attributed to the immune stimulation of LDC@ZnP-M NPs to DCs. Furthermore, to find out the stimulation of LDC@ZnP-MA NPs on DC maturation, BMDCs had been incubated with PBS+/-NIR, Free M + A+/-NIR (10 µg/mL melanin, 4 µg/mL Adpgk), equal LDC@ZnP-M NPs + NIR (akin to 385 µg/mL NPs), LDC@ZnP-A NPs, LDC@ZnP-MA NPs + NIR and LPS (1 µg/mL) for 4 h and changed with contemporary medium. For the teams with NIR irradiation, the cells had been uncovered to 2 W/cm2 laser for two min. After one other 20 h, the cells had been collected and stained with DC (CD11c) and maturation maker (CD86) and detected by stream cytometry. As proven in Fig. 3D, E, LPS can considerably up-regulate the proportion of CD11c+CD86+ matured DCs. In contrast with Free M + A+/-NIR, LDC@ZnP-M NPs + NIR mediated photothermal remedy or LDC@ZnP-A NPs-mediated immunotherapy, LDC@ZnP-MA NPs + NIR-mediated combinational remedy had a big stimulatory impact on DC maturation. Accompany with maturation course of, DCs are liable to secret immunostimulatory cytokines to the extracellular setting. Right here the supernatant was centrifuged to eliminate the cells and particles and measured by ELISA kits. Interleukin 6 (IL-6) is obligated to induce T cell-mediated autoimmunity [36]. Tumor necrosis factor-alpha (TNF-α) is a mediator in mobile immunity, which may promote the cross-presentation by DCs [37]. As proven in Fig. 3F, G, secretion ranges of IL-6 and TNF-α had been remarkably enhanced in LDC@ZnP-MA NPs + NIR handled teams. The outcomes indicated that LDC@ZnP NPs will be successfully uptake and processed by DCs.
The in vitro interplay of LDC@ZnP NPs and BMDCs. A Confocal photographs of BMDCs after incubation with L@ZnP NPs and LDC@ZnP NPs. Cell nuclei had been stained with DAPI, scale bar = 50 μm. B Quantitative uptake of L@ZnP NPs and LDC@ZnP NPs by BMDCs detected by stream cytometer (n = 3), ns: not vital, ***p < 0.001, ****p < 0.0001. C The OD values of BMDCs incubated with completely different quantities of LDC@ZnP-MA NPs (n = 4). D Circulation scatter plots of CD11c+CD86+ cells. E Quantitative evaluation of CD11c+CD86+ cells (n = 3), *p < 0.05. F The extent of IL-6 within the supernatant of BMDCs (n = 3), *p < 0.05. G The extent of TNF-α within the supernatant of BMDCs (n = 3), *p < 0.05
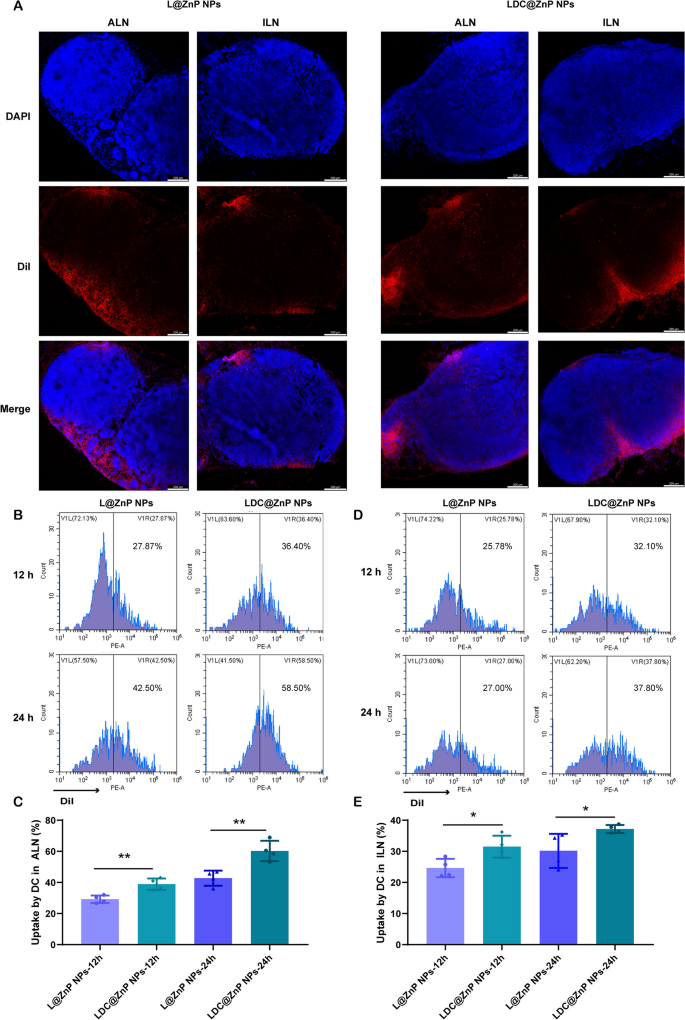
Lymphatic reflux of LDC@ZnP NPs
It has been evidenced that the in vivo biodistribution of nanoparticles is expounded with properties of nanoparticles together with the elemental-chemical compositions, dimension distribution, form, floor hydrophobicity, agglomeration state, optical properties, and so forth. [38, 39]. Usually, the drug loading within the core of nanoparticles with core-shell construction has negligible affect to those properties, resulting in the same biodistribution habits of drug-loaded nanoparticles with clean nanoparticles. Herein, L@ZnP NPs and LDC@ ZnP NPs had been used to observe the in vivo biodistribution. TDLNs play a big function in most cancers immunotherapy, particularly for vaccines. As inguinal and axillary lymph nodes are an important TDLNs after subcutaneous injection of nanovaccine [40], DiI labeled L@ZnP and LDC@ZnP NPs had been then injected subcutaneously into the axillary and inguinal areas of C57BL/6 mice, respectively. After 24 h, the mice had been sacrificed for the gathering of ALNs and ILNs. The tissues had been ready for frozen sections and stained with DAPI. As proven in Fig. 4A and Extra file 1: Fig. S5, the crimson fluorescence of LDC@ZnP NPs was stronger in each ALNs and ILNs compared with L@ZnP NPs. Curiously, for each L@ZnP NPs and LDC@ZnP NPs, the fluorescence depth and distribution within the ALNs had been enhanced than these in ILNs. To additional consider the cell uptake habits by DCs in LNs, ALNs and ILNs had been collected for stream cytometry evaluation after subcutaneous administration of DiI labeled LZnP and LDC@ZnP NPs. At 12 and 24 h post-injection, the lymphocytes in LNs had been stained with CD11c. As proven in Fig. 4B–E, with the extension of injection time, the uptake of each nanoparticles by DCs had been clearly elevated. In contrast with DCs in ILN, DCs in ALN uptake extra L@ZnP NPs and LDC@ZnP NPs. Much like the in vitro uptake habits by BMDCs, LDC@ZnP NPs confirmed enhanced internalization by each ALN and ILN DCs than LZnP NPs. Together with the distribution and uptake of LDC@ZnP NPs, ALNs might be extra appropriate because the tumor-draining lymph nodes.
Lymphatic reflux of LDC@ZnP NPs. A Distribution of L@ZnP NPs and LDC@ZnP NPs in ALNs and ILNs, scale bar = 200 μm. B Uptake of L@ZnP NPs and LDC@ZnP NPs by DCs in ALNs. C Quantitative evaluation of L@ZnP NPs and LDC@ZnP NPs uptake behaviors by DCs in ALNs (n = 4), **p < 0.01. D Uptake of L@ZnP NPs and LDC@ZnP NPs by DCs in ILNs. E Quantitative evaluation of L@ZnP NPs and LDC@ZnP NPs uptake behaviors by DCs in ILNs (n = 4), *p < 0.05
Biodistribution of LDC@ZnP NPs
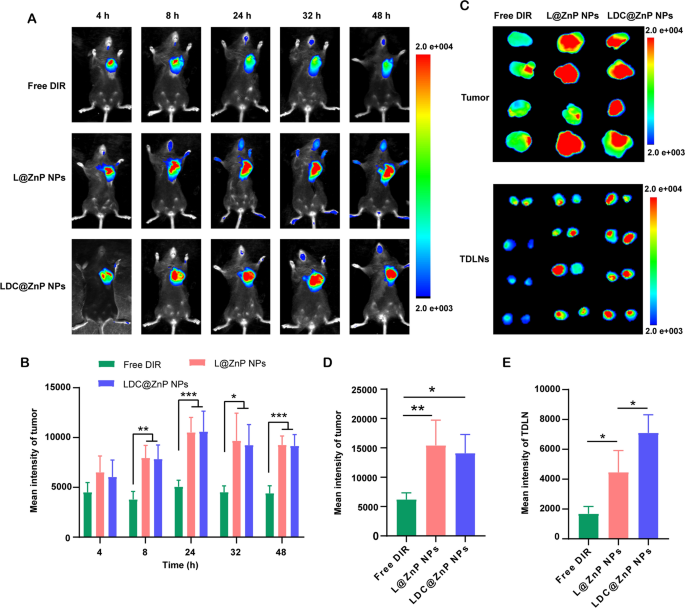
MC38 cells had been inoculated on the proper flank of feminine C57BL/6 mice. When the tumor quantity reached round 500 mm3, the mice had been randomly divided into three teams. The near-infrared dye DIR, DIR-labeled L@ZnP or LDC@ZnP NPs had been injected subcutaneously to the tumor-bearing mice to trace the in vivo biodistribution. Fluorescence photographs had been monitored at completely different time factors (4, 8, 24, 32, 48 h). Completely different with wholesome blood vessels, the blood vessels surrounding tumor are normally discontinuous and irregular owing to the extreme secretion of angiogenic elements by tumor. These malformed blood vessels are lack of basement membrane, thereby displaying elevated permeability with enlarged intercellular gaps [41]. Furthermore, a dynamic blood vessel bursts occurred in tumor blood vessels, which allowed the outflow fluid extravasate into the interstitial house of tumor [42]. As proven in Fig. 5A, the distribution of L@ZnP NPs and LDC@ZnP NPs in tumors elevated throughout 4 to 24 h, reached a peak at 24 h, and maintained a comparatively robust fluorescence depth at 48 h. Fluorescence quantitative outcomes confirmed that there was no vital distinction within the fluorescence depth between L@ZnP NPs and LDC@ZnP NPs in tumors (Fig. 5B). The supply of NPs to tumor might be attributed to the formation of large-size gaps in addition to the dynamic vascular vent in tumor blood vessels. At 48 h post-injection, the mice had been sacrificed. The tumor tissues and TDLNs had been stripped for ex vivo near-infrared imaging. As proven in Fig. 5C, D, the ex vivo fluorescence of tumor was in line with the in vivo outcomes. Notably, LDC@ZnP NPs handled group confirmed elevated fluorescence depth in TDLNs than L@ZnP NPs handled group (Fig. 5E), additional indicating the lymphatic reflux impact of LDC@ZnP NPs in vivo. Furthermore, the key tissues, together with coronary heart, liver, spleen, lung and kidney, had been additionally dissected. As offered in Extra file 1: Fig. S6, Free DIR, L@ZnP NPs and LDC@ZnP NPs had been distributed in all tissues whereas displaying a significant distribution within the livers with wealthy blood provide, indicating that LDC@ZnP NPs will be absorbed subcutaneously into the bloodstream.
Biodistribution of LDC@ZnP NPs in vivo. A In vivo photographs of Free DIR, L@ZnP NPs and LDC@ZnP NPs handled mice at completely different time factors. B Fluorescence quantization of in vivo tumors at completely different time factors (n = 4), *p < 0.05, **p < 0.01, ***p < 0.001. C Ex vivo photographs of Free DIR, L@ZnP NPs, and LDC@ZnP NPs handled tumors and TDLNs at 48 h publish injection (n = 4). D Fluorescence quantization of ex vivo tumors at 48 h publish injection (n = 4), *p < 0.05, **p < 0.01. E Fluorescence quantitation of Free DIR, L@ZnP NPs and LDC@ZnP NPs in ex vivo TDLNs (n = 4), *p < 0.05
In vivo antitumor impact mediated by LDC@ZnP NPs
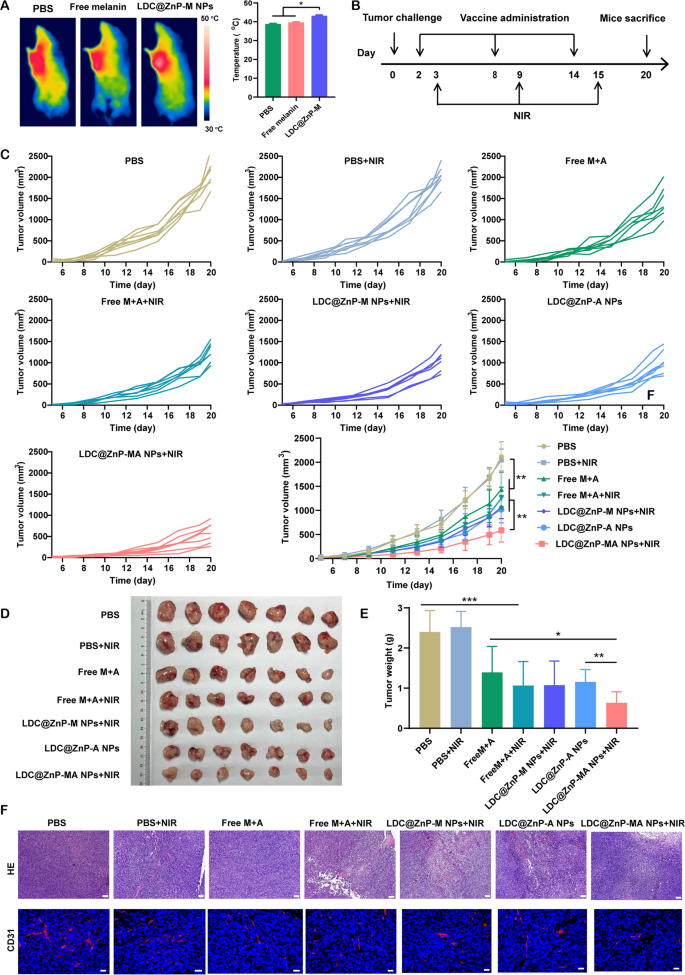
Previous to investigating the antitumor effectivity of LDC@ZnP NPs, the in vivo photothermal impact was decided in tumor-bearing C57BL/6 mice. The mice had been administered with PBS, Free melanin, and LDC@ZnP-M NPs. After 24 h, the mice in every group had been anesthetized with pentobarbital sodium and irradiated with 808 nm laser. The temperature of LDC@ZnP-M NPs handled tumors may enhance to round 42 oC inside 2 min whereas that of PBS and Free melanin had been decrease than 40 oC (Fig. 6A). To additional decide the influence of peptide coloading on the heating impact of NPs, the in vivo photothermal impact of LDC@ZnP-MA NPs had been evaluated after subcutaneous injection. The corresponding quantities of melanin and Adpgk peptide had been 50 µg and 20 µg, respectively. As Extra file 1: Fig. S7 displayed, LDC@ZnP-MA NPs can achieved roughly 42 ℃ after irradiation for two min (2 W/cm2), which had been comparable as LDC@ZnP-M NPs. Then the in vivo antitumor impact of various therapies was investigated. 1.25 × 105 MC38 cells had been inoculated into the best frank of C57BL/6 mice. PBS, PBS + NIR, Free M + A (50 µg melanin and 20 µg Adpgk), Free M + A + NIR, LDC@ZnP-M NPs + NIR, LDC@ZnP-A NPs, LDC@ZnP-MA NPs + NIR got to the tumor-bearing mice, respectively. The vaccination and irradiation adopted the protocol in Fig. 6B. The psychological and physiological circumstances in addition to physique weight had been monitored. Through the therapeutic interval, the physique weight of all handled mice confirmed an elevated tendency (Extra file 1: Fig. S8A), indicating the biosafety of LDC@ZnP NPs. In the meantime, tumor development was monitored by recording the width and size of tumor. As offered in Fig. 6C, the tumor development of PBS + NIR handled mice had been comparable with PBS, demonstrating the photothermal security of NIR in vivo. In contrast with Free M + A and Free M + A + NIR handled mice, the antitumor impact of LDC@ZnP NPs was extra apparent. LDC@ZnP-M NPs + NIR or LDC@ZnP-A NPs mediated immunotherapy displayed comparable tumor suppression. Notably, the mix of peptide and mile warmth impressed immunotherapy mediated by LDC@ZnP-MA NPs + NIR confirmed the very best tumor inhibition efficiency amongst all therapies. Consisting with the outcomes of tumor quantity, the excised tumor picture (Fig. 6D) and tumor mass (Fig. 6E) additionally evidenced the efficient antitumor exercise of LDC@ZnP-MA NPs. Moreover, the tumor inhibitory charges had been calculated against this with PBS. Accordingly, LDC@ZnP-MA NPs handled mice offered the very best tumor inhibitory fee of 73.5% (Extra file 1: Fig. S8B). Afterwards, HE and CD31 immunofluorescent staining of tumor sections was performed to higher discover the antitumor outcomes of LDC@ZnP NPs. As displayed with the most important areas of tumor lysis and necrosis, LDC@ZnP-MA NPs confirmed superior therapeutic impact amongst all therapies. CD31, extremely expressed on endothelial cells, is properly established to observe the vessel density in malignant tissues [43]. As proven in Fig. 6F, a discount of CD31 expression was noticed in LDC@ZnP NPs handled tumors.
In vivo antitumor results of LDC@ZnP NPs. A The in vivo photothermal photographs (left) and temperature (proper) after irradiating with 808 nm laser for two min (n = 3), *p < 0.05. B Time schedule of remedy. C Tumor development curves of MC38 handled by completely different formulations (n = 7), *p < 0.05, **p < 0.01. D Photographs of dissected tumors (n = 7). E Tumor weight in every group (n = 7), *p < 0.05, **p < 0.01, ***p < 0.001. F HE and CD31 staining photographs of tumor tissue after completely different therapies, scale bars had been 200 nm for HE and 20 nm for CD31 staining, respectively
The antitumor immune responses elicited by LDC@ZnP NPs
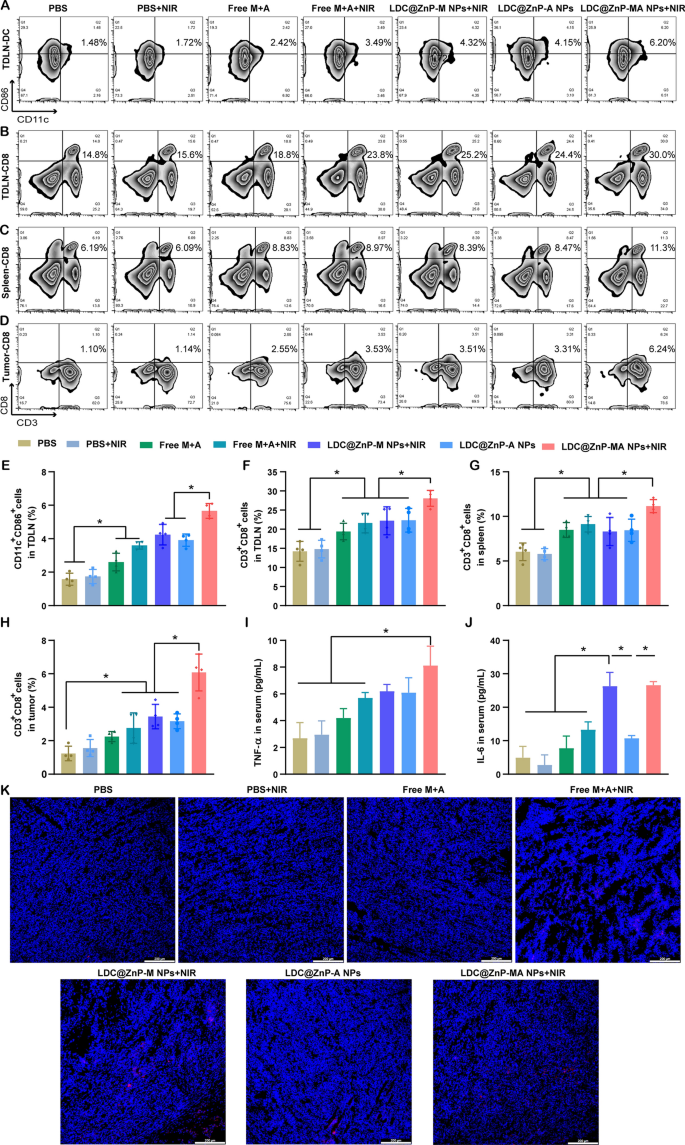
To discover the underlying mechanism of LDC@ZnP NPs mediated immunotherapy, we analyzed the immune cells and cytokines after 48 h of final injection. TDLNs are correlated with antitumor immunity as a result of particular location and roles. We now have evidenced that LDC@ZnP NPs can successfully reflux to TDLNs and be uptake by DCs. As DC maturation was important for antigen presentation, right here we firstly analyzed the in vivo DC maturation after vaccination by completely different formulations. As proven in Fig. 7A and E, the matured DCs in PBS + NIR, Free M + A, Free M + A + NIR, LDC@ZnP-M NPs + NIR, LDC@ZnP-A NPs, LDC@ZnP-MA NPs + NIR handled TDLNs had been 1.1-, 1.7-, 2.3-, 2.7-, 2.5- and three.6-fold in contrast with PBS, respectively. Notably, LDC@ZnP-MA NPs + NIR offered the very best DC maturation amongst all teams, which might be helpful to induce subsequent antitumor immune response. As an necessary lymphoid tissue for systematic antitumor immunity, the DC maturation of spleen was additionally analyzed, which confirmed comparable outcomes as TDLNs (Extra file 1: Fig. S9). It was additionally discovered that DC infiltrated in tumor microenvironment, and LDC@ZnP-MA NPs + NIR handled teams elevated DC inhabitants in contrast with the management group (Extra file 1: Fig. S10). After maturation, DCs would current the tumor related antigens to T cells to elicit particular antitumor immunity [44]. As a vital part of T cells, cytotoxic lymphocytes (CTLs, CD3+CD8+ cells) play a necessary function in antitumor immunotherapy [45, 46]. Right here, we analyzed CTLs in TDLNs, spleen, and tumor tissues. In TDLNs, the proportion of CTLs for PBS, PBS + NIR, Free M + A, Free M + A + NIR, LDC@ZnP-M NPs + NIR, LDC@ZnP-A NPs and LDC@ZnP-MA NPs + NIR had been 14.2 ± 2.6%, 14.8 ± 2.36%, 19.4 ± 2.2%, 21.6 ± 2.6%, 22.2 ± 3.7%, 22.3 ± 3.1% and 28.1 ± 2.1%, respectively (Fig. 7B, F). In the meantime, CTLs in spleen of PBS, PBS + NIR, Free M + A, Free M + A + NIR, LDC@ZnP-M NPs + NIR, LDC@ZnP-A NPs and LDC@ZnP-MA NPs + NIR handled mice had been 6.0 ± 1.0%, 5.8 ± 0.6%, 8.5 ± 0.8%, 9.1 ± 0.9%, 8.3 ± 1.6%, 8.4 ± 1.2% and 11.2 ± 0.7%, respectively (Fig. 7C and G). For tumor microenvironment, the infiltration of CD3+CD8+ cells in LDC@ZnP-MA NPs + NIR handled group was 4.9-, 3.9-, 2.7-, 2.2-, 1.8-, and 1.9-fold than in PBS, PBS + NIR, Free M + A, Free M + A + NIR, LDC@ZnP-M NPs + NIR, LDC@ZnP-A NPs handled teams, respectively (Fig. 7D and H). CD4+ helper T cells, an necessary subset of T cells, promote each the effector and reminiscence capabilities of CTLs and assist CTLs to beat unfavorable rules [47]. After vaccination by LDC@ZnP-MA NPs + NIR, the proportion of CD3+CD4+ cells had been remarkably enhanced in contrast with different therapies (Extra file 1: Fig. S11, S12). Furthermore, the discharge of immunostimulatory cytokines after completely different vaccinations was decided by ELISA. As offered in Fig. 7I, J, the serum ranges of TNF-α and IL-6 confirmed the very best secretion in LDC@ZnP-MA NPs + NIR handled mice amongst all of the teams. The immunofluorescent staining of IL-6 in tumor sections displayed comparable outcomes (Fig. 7Ok). These outcomes advised that the mix of tumor related antigen and delicate warmth impressed immunotherapy may induce efficient antitumor immune responses.
The antitumor immune responses elicited by LDC@ZnP NPs. The mice had been handled with PBS, PBS + NIR, Free M + A, Free M + A + NIR, LDC@ZnP-M NPs + NIR, LDC@ZnP-A NPs and LDC@ZnP-MA NPs + NIR, respectively. A Circulation scatter plots of CD11C+CD86+ cells in TDLNs. B Circulation scatter plots of CD3+CD8+ cells in TDLNs. C Circulation scatter plots of CD3+CD8+ cells in spleens. D Circulation scatter plots of CD3+CD8+ cells in tumor tissues. E Quantitative evaluation of CD11C+CD86+ cells in TDLNs (n = 4), *p < 0.05. F Quantitative evaluation of CD3+CD8+ cells in TDLNs (n = 4), *p < 0.05. G Quantitative evaluation of CD3+CD8+ cells in spleens (n = 4), *p < 0.05. H Quantitative evaluation of CD3+CD8+ cells in tumors (n = 4), *p < 0.05. I Serum ranges of TNF-α (n = 3), * p < 0.05. J Serum ranges of IL-6 (n = 3), *p < 0.05. Ok Immunofluorescent staining of IL-6 in tumor sections. The nuclei had been stained with DAPI, scale bar = 200 μm