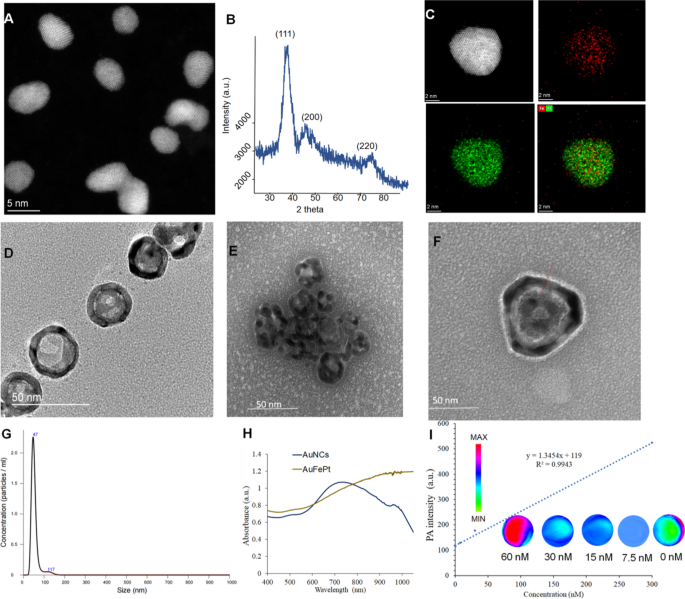
Preparation and characterization of FePt, AuNCs and Au@FePt NPs
The FePt and AuNCs NPs had been synthesized utilizing the literature methodology with modifications, and a sequence of experiments had been performed to find out the morphology of FePt, AuNCs and Au@FePt NPs, reminiscent of TEM, XRD and DLS. The morphologies revealed by high-resolution TEM (HRTEM) for FePt and Au@FePt had been proven in (Fig. 1A) which exhibits the uniform form of FePt nanoparticles, which had been roughly 4 nm in dimension. Determine. 1B revealed that every one three diffraction peaks had been listed to FePt. Elemental mapping of single and a number of FePt particles is proven in Fig. 1C which demonstrated Fe and Pt species introduced within the nanoparticles. The TEM picture of AuNCs is proven in Fig. 1D to find out the floor morphology of the cage’s current gap to load small molecules and HRTEM pictures was proven in Extra file 1: Fig. S2. Determine 1E exhibits a excessive payload of FePt in AuNCs for dual-mode imaging as exogenous distinction brokers. The intrinsic properties of Au@FePt NPs had been investigated in HRTEM with the loading of FePt NPs revealing a morphology with a diameter of ~ 4 nm inside the outlet of the AuNCs. The particle dimension and distribution by DLS of Au@FePt in Fig. 1G revealed that the dimensions of Au@FePt barely elevated (roughly 40 nm). The absorption spectra of AuNCs and Au@FePt NPs are introduced, indicating the encapsulation course of didn’t have an effect on the optical properties of AuNCs and FePt to function wonderful photothermal brokers. Apart from, the absorption of Au@FePt NPs displayed a broad peak centered at 900 nm in pH 6.5 phosphate buffer resolution (PBS) (Fig. 1H) which indicated the native floor plasmon resonance (LSPR) broadens the NIR absorption area. To additional consider the homogeneity and dispersibility of Au@FePt in numerous pH buffers upon laser irradiation [43], the change in hydrodynamic radius of Au@FePt was recorded for 1 day as proven in Extra file 1: Fig. S3. The outcome suggests the Au@FePt nanoprobe could possibly be an appropriate candidate below regular physiological situation for drug supply functions.
A The TEM pictures and B the powder X-ray diffraction (PXRD) evaluation of FePt nanoparticles (scale bar = 5 nm). C The STEM-EDS elemental mapping pictures and D the TEM pictures of AuNCs nanoparticles. E The TEM pictures of Au@FePt nanoparticles. F The excessive decision TEM of 1 gold nanocage loaded FePt. G Hydrodynamic dimension distribution of Au@FePt nanoparticles. H UV–Vis spectrum of Au@FePt and AuNCs nanoparticles. I PA depth of Au@FePt NPs in numerous concentrations
Photoacoustic and photothermal properties of the Au@FePt NPs
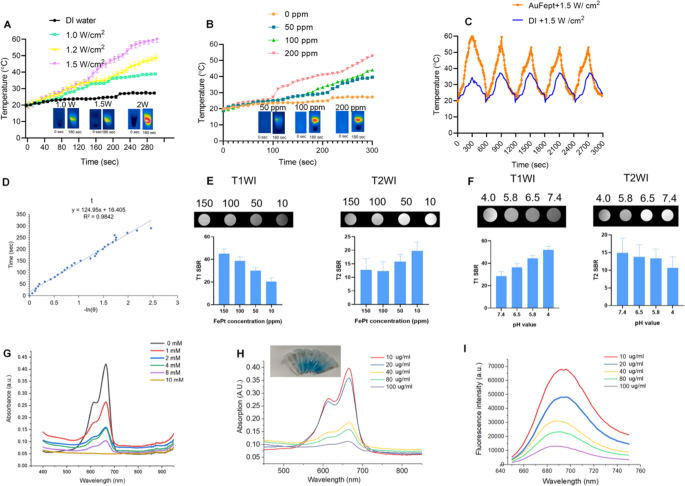
As proven in Fig. 1I, the Au@FePt NPs exhibit intensive PAI alerts upon irradiation at 1064 nm captured by the Vevo 2100 animal PAI system, and their PA alerts elevate linearly with the Au@FePt concentrations. The excessive PAI sensitivity was primarily because of the conspicuous absorption of Au@FePt NPs at 1064 nm. Photothermal-heating curves of Au@FePt NPs and corresponding thermal pictures had been exhibited to reveal that Au@FePt NPs are possible for photothermal brokers [44]. Moreover, after 1064 nm laser irradiation, the temperature elevation of Au@FePt NPs in aqueous resolution elevated in a laser power-dependent method (from 1.0 to 1.5 W/cm2), as proven in Fig. 2A. As proven in Fig. 2B, the temperature for Au@FePt NPs elevated significantly with the rise in AuNCs’ focus. In distinction, the temperature of the management group’s deionized (DI) water confirmed negligible adjustments at 1.2 W/cm2, which demonstrated that the Au@FePt NPs might convert NIR-II gentle vitality into thermal vitality adequately [45]. As well as, only a negligible change was observed after three repeated heating cycles upon 1064 nm laser on/off (1.5 W/cm2) of Au@FePt NPs as indicated in Fig. 2C. Considerably, the AuNCs contributed wonderful photothermal conversion properties to to Au@FePt NPs which affords good proof for in vivo PTT experiments. The photothermal conversion effectivity worth (η) was 31.3% as proven in Fig. 2D which was a bit larger than beforehand reported nanoparticles [46]. These outcomes confirmed that this Au@FePt nanoprobe has considerably excessive photothermal conversion means and is a perfect photothermal agent for twin imaging guided gentle PTT mixed with CDT. Au@FePt nanoprobes containing 1 mg Fe had been dispersed in 1 mL HNO3 resolution (pH = 5.8, 7.4, with or with out laser) in a dialysis tubing bag and the filtrates had been collected for Fe focus quantification with ICP-OES with at 24-h intervals. The outcomes indicated ~ 18.9%, ~ 8.2% (with laser irradiation) ~ 6.2% and a couple of.4% (with out laser irradiation) Fe launch of Au@FePt nanoparticles below numerous pH circumstances with and with out laser for twenty-four h (Extra file 1: Fig. S4).
A Temperature elevation curves of Au@FePt NPs (100 ppm) below totally different laser powers. B Temperature adjustments curve of Au@FePt NPs (100 ppm) in numerous concentrations throughout 5 min laser irradiation at 1.2 W/cm2. C A number of heating cycles of Au@FePt NPs (100 ppm) (orange plot) and DI water (blue plot). D The connection between the primary cooling time and the destructive pure logarithm of the temperature-driving power was calculated from Au@FePt nanoparticles. E In vitro T1 and T2 signal-to-background (SBR) of Au@FePt in numerous focus. F T1 and T2 SBR of Au@FePt nanoprobes incubated at numerous pH. G Methylene blue (MB) (10 μg/mL) degraded by ·OH generated from Au@FePt nanoprobes (Fe focus: 100 µg/mL) and totally different concentrations of H2O2 (0, 2, 4, 8, and 10 mM). H MB absorbance after incubation with Au@FePt nanoprobes on the manufacturing of ·OH, with gradient Fe concentrations (0, 10, 20, 40, 80, 100 μg/mL) and H2O2 (10 mM) was measured. I MB fluorescence spectra after incubation with numerous concentrations of Au@FePt nanoprobes (0, 10, 20, 40, 80, 100 μg/mL) and H2O2 (10 mM) had been measured
Responsive MRI sign of the Au@FePt NPs
The outcomes from Fig. 2E confirmed that the MRI sign was considerably extra delicate for detecting injected phantoms that are a optimistic T1-weighted imaging (T1WI) enhancer when releasing Fe2+. Furthermore, the paramagnetic Fe atoms that stay within the undissociated FePt probes are wonderful destructive T2WI distinction brokers (Fig. 2E), akin to a latest report, whereas the launched Fe2+ might be acted as a optimistic T1 WI distinction agent. Based mostly on 7.0 T MRI knowledge, the T2-weighted rest charge of Au@FePt was 79.361 s−1 and the T1-weighted rest charge was 4.027 s−1, emphasizing the competence of the Au@FePt nanoprobe as T1/T2-weighted dual-mode MRI distinction agent, elevating the potential for a way to exactly establish the tumor area to be detected [37]. By way of the MRI switching impact, the paramagnetic Fe atoms that stay within the undissociated Au@FePt are wonderful destructive T2WI distinction brokers, whereas the launched Fe2+ is a optimistic T1WI enhancer. As proven in Fig. 2F, the T2-signal-to-background (SBR) in a impartial situation (pH = 7.4) was 14.9, decrease T2-SBR values had been noticed when Au@FePt NPs had been uncovered to acidic circumstances, with values of 13.8, 13.3 and 10.64, decided at pH 6.5, 5.8, and 4.0, respectively.
In vitro check for Fenton response
By way of the Fenton response, H2O2 is transformed into extremely poisonous ·OH, which is catalyzed by Fe2+. The generated ·OH can effectively disrupt the important thing features of a most cancers cell and its constructions of biomolecules, reminiscent of DNA, proteins, and lipids [47]. The methylene blue (MB) because the dye might be decolored by OH, which was utilized to disclose the ·OH era means of Au@FePt through a Fenton response [36]. Determine 2G confirmed that the absorbance of MB dramatically declined after remedy with Au@FePt nanoprobes (Fe focus: 100 µg/mL) and totally different concentrations of H2O2 (0, 2, 4, 8, and 10 mM) (100 μg/mL), indicating an H2O2-dependent ·OH era. Moreover, MB absorbance within the Au@FePt resolution step by step decreased with growing Au@FePt focus (from 0 to 100 μg/mL), indicating a considerable amount of ·OH era after the Fenton response from Fe2+ below acidic circumstances (Fig. 2H). Notably, the depth of the MB fluorescence step by step decreased because the Au@FePt focus elevated from 0 to 100 mg/mL (Fig. 2I). Total, the H2O2 concentration-dependent Fenton impact of Au@FePt might additional improve ·OH era.
In vitro cytotoxicity assay of the Au@FePt NPs
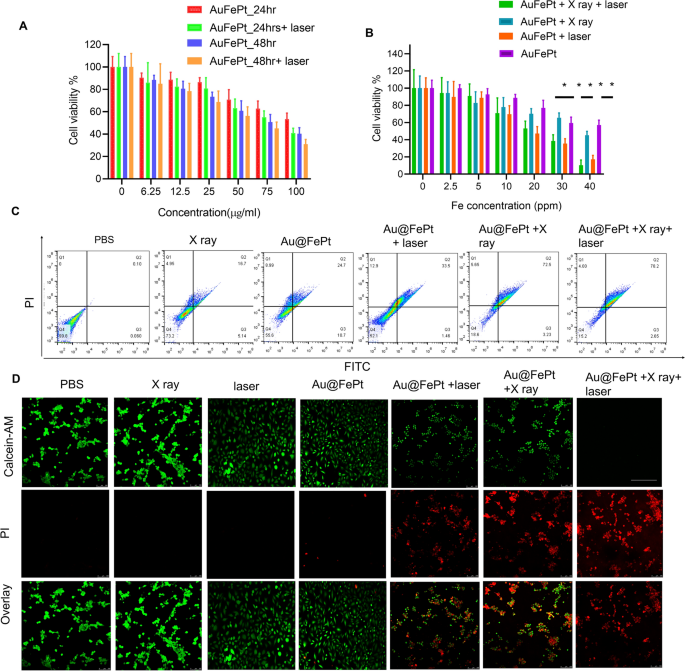
The in vitro cytotoxicity of Au@FePt NPs with photothermal/radiation synergetic remedy in opposition to 4T1 cells below totally different remedy instances was assessed by the usual CCK-8 assay. As proven in Fig. 3A, the cytotoxicity outcome revealed that the Au@FePt + laser (1.2 W/cm2, 5 min) group’s cytotoxicity (1.2 W/cm2, 5 min) was 1.2 and a couple of.6-fold larger than that of Au@FePt teams at 24 and 48 h, respectively. As proven in Fig. 3A, the IC50 worth (i.e., the drug concentrations required to induce 50% cell demise inside a sure interval) of Au@FePt with out laser irradiation was 150 μg/mL and 109.7 μg/mL after 24 and 48 h of incubation, respectively. In comparison with the IC50 of Au@FePt receiving laser irradiation (108.9 μg/mL and 74.5 μg/mL) after 24 h and 48 h incubation, the cell viability of Au@FePt notably diminished, demonstrating that the AuNCs encapsulation of FePt exhibited larger cytotoxicity upon 1064 nm laser irradiation (1.2 W/cm2, 5 min). Apart from, the cytotoxicity was ranked as follows: Au@FePt + NIR + X-ray > Au@FePt + laser > Au@FePt + X-ray > Au@FePt in Fig. 3B. The information indicated that the Au@FePt with out laser and X-ray publicity revealed comparatively weaker cytotoxicity on most cancers cells. Remedy with Au@FePt below laser and X-ray publicity resulted in larger cytotoxicity in direction of 4T1 cells than Au@FePt + laser and Au@FePt + X-ray teams, indicating that gentle PTT and XDT induced larger cytotoxicity in most cancers cells, as proven in Fig. 3C. Circulation cytometry was used to research the cell demise pathways of Au@FePt below numerous therapies in comparison with management teams after staining with an annexin V-FITC/PI package. Au@FePt with laser and X-ray had the strongest CDT means below gentle PTT and will induce early apoptosis accompanied by secondary necrotic/late apoptosis (78.5%), in step with cell viability assay outcomes (Fig. 3C). The outcomes confirmed that the apoptotic charges of the PBS group, X ray, Au@FePt, Au@FePt + laser, Au@FePt + X rayand Au@FePt + X-ray teams had been ~ 0.1%, ~ 16.7%, ~ 24.7%, ~ 33.5, 72.5% and 78.2%, respectively (Fig. 3D), which had been much like these of the CCK8 assay.
A The cell viability of 4T1 cells after remedy with totally different concentrations of Au@FePt for twenty-four h and 48 h (n = 5). B Cell viability check of Au@FePt and with and with out X-ray or laser irradiation for twenty-four h (n = 5). Knowledge symbolize imply ± SD from three impartial experiments, every carried out in 5 replicates (*P < 0.05, **P < 0.01). C Circulation cytometry analyses of cell apoptosis by Annexin V-FITC/PI co-staining of various remedy teams. D Calcein-AM/PI co-staining of 4T1 cells after the indicated therapies (inexperienced: dwell cells; purple: useless cells) of assorted handled teams (scale bar = 100 μm)
As well as, the remedy impact was additional confirmed by the dwell/useless cell staining assay was performed utilizing a calcein-AM/PI package to additional confirm the synergetic low-temperature PTT with XDT/CDT impact of Au@FePt. Beneath gentle irradiation for five min (1064 nm, 1.2 W/cm2), Au@FePt + laser and X-ray induced roughly 90% cell demise, which was in step with CCK8 outcomes (Fig. 3D). The cell imaging knowledge demonstrated that the whole thing of the cell nuclei within the Au@FePt + laser + X-ray group had a robust PI sign associated to chromatin condensation, indicating that cell demise had occurred in comparison with the X-ray with or with out laser irradiation as proven above [48]. Though the killing effectivity of most cancers cells through Au@FePt was tremendously weakened with out X-ray remedy, the PTT and CDT results of Au@FePt NPs had been maintained, additional highlighting the necessary position of Au@FePt NPs in CDT-combined gentle PTT. Furthermore, with out the usage of a laser, fluorescence cell imaging revealed that the XDT/CDT consecutively broken the cell membrane and induced apoptosis within the Au@FePt + X-ray teams.
Transcriptomic signatures by means of RNA sequencing by Au@FePt remedy in breast most cancers
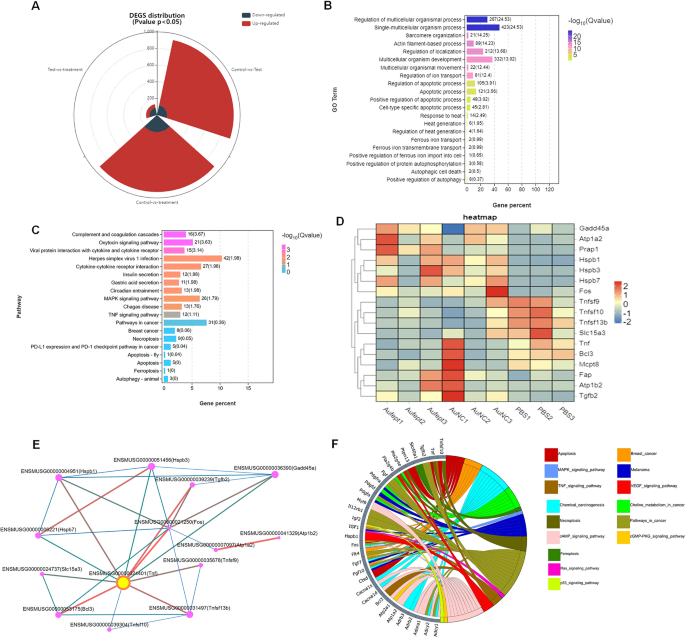
To guage the transcription profile in Au@FePt handled, the RNA sequencing evaluation of the 4T1 tumor mannequin with totally different therapies was carried out. The distribution of differentially expressed genes (DEGs) quantity between totally different teams was proven as a stacked-rose chart (Fig. 4A). Provided that the Au@FePt synergetic remedy resulted within the biggest change in DEG quantity when in comparison with different handled teams, we investigated the potential practical and mechanism pathways enriched within the Au@FePt + laser + X-ray group’s DEGs. The gene ontology (GO) enrichment evaluation confirmed that the DEGs expression of Au@FePt + laser + X-ray remedy was sometimes enriched within the organic processes, involving “Response to warmth”, “Warmth era”, “Apoptosis course of”, “Regulation of iron transport, and “Constructive regulation of apoptosis” (Fig. 4B). Moreover, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment evaluation revealed a number of pathways associated to most cancers pathology that had been regulated by the AuFeT + laser + X-ray remedy (Fig. 4C). Moreover, a heatmap was plotted to depict the distinct expression patterns of 18 consultant DEGs amongst totally different handled teams (Fig. 4D). Up- and down-regulated genes in every group in contrast with these of the PBS had been set to GO enrichment evaluation. To additional characterize the relational hyperlinks of those consultant genes, a protein–protein interplay (PPI) community indicated the relational associations amongst encoded genes. The dimensions and colour of the rings symbolize the significance of various genes within the PPI community. Leads to PPI point out the hub gene was TNF, which encodes the tumor necrosis factor-alpha that has been a vital think about tackling breast most cancers (Fig. 4E). In-depth, to visualise relations between genes and pathways, we offer a Circos plot to depict the subordination of 32 consultant DEGs. The plot exhibits that the “cAMP signaling pathway” and “most cancers pathway” have the very best variety of genes enriched. Moreover, different pathways, together with “MAPK signaling pathway,” “TNF signaling pathway,” “Apoptotic pathway,” “Pathway in cell demise,” “Ferroptosis pathway,” “Ras signaling pathway,” and “P53 signaling pathway” additionally participated within the gene expression change of Au@FePt + laser + X-ray remedy (Fig. 4F). To be temporary, an intensive variety of DEGs had been recognized after Au@FePt + laser + X-ray remedy, and Au@FePt + laser + X-ray might trigger transcriptional dysregulation and have an effect on oncogenic signaling in 4T1 tumor cells, particularly in apoptosis and ferroptosis. Bioinformatic outcomes revealed that biomineralization might tremendously inhibit most cancers development by disrupting the warmth era course of and inducing cell cycle arrest, apoptosis, and ferroptosis.
RNA-seq and differentially expressed genes (DEGs) outcomes. RNA sequencing of 143B cells after remedy of regular saline as Management, Ca, DPA, or DPAC. A The panorama of up-and-down regulated DEGs quantity distributed between teams that examine with one another (PBS + laser vs Au@FePt + laser + X-ray, PBS + laser vs AuNCs + laser, AuNCs + laser vs Au@FePt + laser + X-ray). log2FC > 1.5 was outlined as upregulation (purple) and log2FC < − 1.5 was outlined as downregulation (gray-blue). The panorama of DEGs distribution between management (PBS remedy) and Au@FePt + laser + X-ray teams. Coloured factors symbolize FDR < 0.05 (− log (FDR) ≥ 1.5, dashed line), log2FC > 1.5 upregulated genes (yellow) and log2FC < − 1.5 downregulated genes (purple). B GO enrichment evaluation of DEGs for the Au@FePt + laser + X-ray handled mice. A Fisher’s precise check displayed significance ranges (− log10 Q-value, dimension). The variety of genes enriched in every GO time period and the Q worth had been marked subsequent to every bar. C Bar plot displaying the highest 19 enriched KEGG pathways for DEGs within the Au@FePt + laser + X-ray handled mice. The variety of genes enriched in every pathway and the Q worth was marked subsequent to every bar. KEGG pathway evaluation of from Au@FePt + laser + X-ray handled mice and PBS handled glioblastoma. enriched p38 mitogen-activated protein kinase (MAPK) signaling pathway and tumor necrosis issue (TNF) signaling pathway. D A heatmap exhibits apoptosis/ferroptosis-related differentially expressed genes between therapies. Coloration depth represents the diploma of expression worth of this gene after standardized remedy in every pattern. Relative expression ranges are proven in purple (up-regulation) and blue (down-regulation). E PPI community demonstrating particular gene interplay pathways derived from consultant DEGs, and yellow nodes represented the places of a hub node. PPI community consists of particular genes encoded by consultant DEGs. F The Circos plot visualizes important associations between 32 consultant DEGs and KEGG pathways in a distinct colour. Icons marked the 15 cancer-relative KEGG pathways enriched by consultant DEGs
Intracellular ROS era
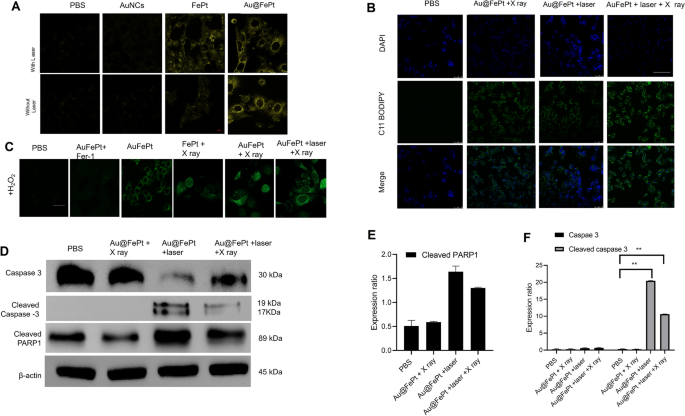
To disclose the in vitro therapeutic efficacy, the promising benefit of AuNCs loaded FePt was attributed to cell internalization on the tumor microenvironment for exactly enhanced apoptosis/ferroptosis results in breast most cancers. The era of Fe2+ was verified utilizing the Ferro Orange fluorescence staining assay, whose fluorescence depth elevated because the Fe2+ was enriched within the cells. Au@FePt NPs handled 4T1 most cancers cells (+NIR-II laser irradiation) demonstrated an enhanced fluorescence sign than the Au@FePt and PBS group (Fig. 5A), indicating that co-incubation with laser enhanced the Fe2+focus in 4T1 cells. Apart from, the BODIPY581/591-C11 probe was added to detect the LPO degree within the 4T1 cell. Fer-1 (a ferroptosis inhibitor) was added to 4T1 cells previous to the BODIPY581/591-C11 assay to straight examine the LPO impact on Au@FePt + laser + X-ray-induced cell demise. In Fig. 5B, the Au@FePt with laser plus X-ray radiation group revealed the strongest inexperienced fluorescence, adopted by the Au@FePt + laser, Au@FePt + X ray, and PBS teams as proven in Fig. 5B. The cell pictures outcome demonstrated that the Au@FePt gentle PTT plus X-ray radiation group produces a large amount of LPO, which was mixed with the ROS manufacturing and GPX4 depletion outcome [35].
A Fe2+ fluorescence staining with out and with FePt nanoprobes remedy in 4T1 cells (scale bar: 50 μm). B Fluorescence pictures of lipid peroxide in 4T1 cells after remedy with PBS, FePt-Fer-1, Au@FePt + NIR, and Au@FePt + NIR + X-ray (scale bar: 50 μm). C Intracellular ROS era was detected by the DCFH-DA probe after numerous therapies in 4T1 cells (Fe focus: 5 mM, X-ray: 4 Gy) (scale bar: 50 μm) (*P < 0.05, **P < 0.01). D Western blot evaluation of apoptosis-associated proteins after 48 h remedy inside totally different nanoprobe-incubated cells. E, F Quantitative measurement of apoptosis-associated proteins in numerous nanoprobe-incubated cells throughout western blot imaging course of (n = 3)
To research the improved ROS era means of the Au@FePt nanoprobe, the change in oxidative stress was investigated utilizing DCFH-DA as a ROS sensor probe, which might be oxidized by ROS into 2′,7′-dichlorofuorescein (DCF), a inexperienced fluorescence-emitting molecule. As illustrated in Fig. 5C, to imitate the tumor microenvironment overexpressing H2O2, 4T1 cells had been incubated with H2O2 whereas present process totally different therapies. In contrast with the PBS group, Au@FePt-treated 4T1 cells exhibited a certain quantity of inexperienced fluorescence, indicating that ROS era was detected, revealing that Fe2+ launched from Au@FePt undergoes a Fenton response within the presence of H2O2 to play a task in CDT. Nonetheless, within the presence of Fer-1, Au@FePt-treated cells exhibited extraordinarily low fluorescence alerts, indicating that Fer-1 inhibited the operate of Fe2+and additional illustrating that it’s the Fe2+ in Au@FePt that performs a task in ROS era. Au@FePt-treated cells confirmed the very best fluorescence sign after publicity to gentle PTT and X-ray radiation, indicating essentially the most ROS era, which illustrated the superb mixed CDT/PTT/XRT therapeutic impact of the nanoprobe. Ferroptosis acts as one of many typical RCD based mostly on Fe-dependent oxidative injury and is induced by the discount of glutathione peroxidase 4 (GPX4). Down-regulation of GPX4 would outcome within the formation of lipid peroxide (LPO) in most cancers cells.
Activated apoptosis and ferroptosis by means of syngenetic remedy
To look at the cell demise mechanism, a western blot was employed to observe the cell demise pathway upon syngenetic remedy, which signifies the most cancers cell pathway through quantitative in situ activation of intracellular apoptosis/ferroptosis-related proteins. Total, the above outcomes strongly confirmed that Au@FePt + laser + X-ray handled cells might inhibit autophagy in most cancers cells, thus sensitizing the Au@FePt-mediated PTT and CDT-mediated syngenetic therapeutic impact.
The RCD mechanism through numerous protein markers of apoptosis was additional assessed by western blot, together with caspase-3, cleaved caspase 3 and cleaved poly (ADP-ribose) polymerase (PARP) [49]. The western blot outcomes revealed that Au@FePt-induced XDT/CDT/PTT upon laser irradiation remedy up-regulated the expression of caspase-3 (Fig. 5D). To disclose the detailed cell demise mechanism of various therapies through the bidirectional crosstalk between apoptosis and ferroptosis, we research the apoptosis habits with NIR-II laser irradiation or with X-ray in numerous teams. Apoptosis was assessed by caspase 3, cleaved caspase-3, and cleaved poly (ADP-ribose) polymerase (PARP). As proven in Fig. 5E, F, the Au@FePt receiving laser with X-ray exhibited the next apoptosis charge than different teams, reminiscent of Au@FePt + laser and Au@FePt + X-ray and PBS management group. Subsequently, PARP-1 was cleaved to symbolize a sign of apoptosis as proven in Fig. 5F. These outcomes demonstrated that each intrinsic and extrinsic apoptotic pathways had been activated by the synergistic photothermal and enhanced chemotherapy therapies [42, 43, 50].
In vivo MRI and PA imaging
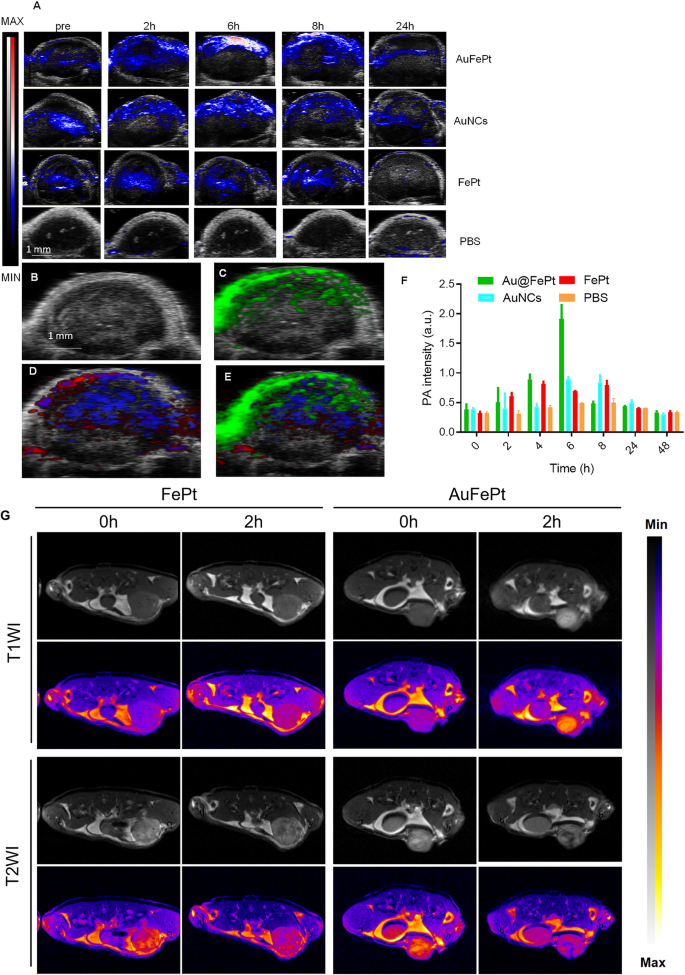
To disclose the in vivo focusing on means of Au@FePt, the FePt and AuNCs NPs (10 mg/kg) had been administrated through tail vein into orthotopic 4T1 tumor-bearing mice. Vevo 2100 photoacoustic system was used to look at the photoacoustic picture and sign adjustments. These PA overlay ultrasound pictures demonstrated a neighborhood distribution of the Au@FePt over 24 h. The PA pictures revealed that the tumor was lightened after injection of Au@FePt nanoprobes at 6 h post-injection which then began to say no and returned to the pre-injection degree on the finish, whereas the PBS management group exhibited minor PA sign change throughout the whole interval. The PA sign within the breast most cancers website of Au@FePt-treated mice elevated over time and reached its highest degree after 6 h post-injection, which achieved a 2.8-fold PA sign enhancement over AuNCs and FePt at 6 h (Fig. 6A). The elevated native focus of Au@FePt in a tumor right now level revealed the affiliation between the endothelial cells based mostly on αvβ3 integrin which is extremely expressed within the anagenesis of the vascular construction ensuing within the nanoparticle focusing on the tumor website straight. The creation of a leaky vasculature, which was characterised as elevated blood circulation across the tumor, might have contributed to the hypoxic core that was seen.The sign from focused Au@FePt might be deconvoluted through PA multispectral scanning with particular absorption spectra for HbO2 and Hb within the tumor area (Fig. 6B–E) [51]. Following intravenous supply, the photoacoustic intensities elevated by an element of two.1, revealing the potential of Au@FePt NPs as succesful photoacoustic distinction brokers for tumor imaging (Fig. 6F).
Monitoring totally different nanoprobe accumulation with photoacoustic imaging. A Warmth map of NP accumulation in tumors in PA-US overlay pictures respectively with totally different nanoprobe into mice bearing 4T1 tumors after 0 h, 2 h, 6 h, 8 h and 24 h (n = 3). B In vivo ultrasound picture of a subcutaneous implanted 4T1 tumor. C Warmth map of PA alerts from focused nanoprobe injected in mice implanted with 4T1 tumor (inexperienced). D Warmth map of oxygenated hemoglobin (purple) and deoxygenated hemoglobin (blue) of the identical 4T1 tumor. E Overlaid with ultrasound picture and focused nanoprobe, oxygenated hemoglobin, and deoxygenated hemoglobin PA alerts. F Common PA depth increment at 850 nm as a operate post-injection time with totally different nanoprobe into mice bearing 4T1 tumors after 0 h, 2 h, 4 h, 6 h, 8 h, 24 h, and 48 h (n = 3). Error bars symbolize normal deviation. *p < 0.05. G In vivo T1WI and T2WI of 4T1 tumors at numerous time factors after intravenous injection of Au@FePt nanoprobe and FePt nanoprobe
Twin imaging with PAI mixed with MRI exhibited superiority each in morphological and practical imaging. Moreover, the efficiency of Au@FePt NPs as distinction brokers for dual-mode in vivo tumor imaging was investigated. Herein, the photographs of T1-weighted and T2-weighted 4T1 tumor-bearing mice had been recorded, and the corresponding sign at totally different time factors was measured. As proven in Fig. 6G, earlier ex vivo MRI switching knowledge revealed that in Au@FePt corrosion, the comfort charge modified from T2 to T1 in an acidic situation. Due to this fact, the similar sections of breast tumor-bearing animals had been imaged utilizing T1 and T2 weighting, and the signal-to-background ratios (SBRs) at numerous time intervals had been computed. After intravenous injection of Au@FePt nanoprobes, the tumors solely confirmed an elevated T1WI sign at tumor places, indicating Fe2+ launch on the tumor websites. Apart from, tumor areas confirmed destructive T2WI enhancement, which is regarded as an indication that Au@FePt have been retained in these tumors. After calculating the ratios of T1 SBR and T2 SBR, it was found that the ratio considerably elevated for 2 hours after injection (Extra file 1: Fig. S7A–C). Apart from, for the FePt (2 mg/mL for Fept; 200 µL) as non-targeted group was administered to the mice bearing 4T1 by means of the tail vein and imaged repeatedly for two days, each 2 h per picture. The outcomes indicated that focused (Au@FePt) present excessive uptake within the tumor website after tail vein injection in comparison with the FePt group (Extra file 1: Fig. S7D–F). Total, the Au@FePt can improve optimistic T1/destructive T2 distinction in MRI and mix with the brand new imaging expertise of photoacoustic imaging to offer a excessive tumor imaging distinction to make sure enough imaging sensitivity. Moreover, PEG-modified phospholipid bilayer shell launch of Au@FePt in a pH-sensitive method gives in situ tumor focusing on and achieves the gentle photothermal/radiation synergetic remedy impact of potentiating ferroptosis impact. In contrast with conventional strategies, multifunctional nanoparticles have benefits that may understand dual-modal new imaging and multi-process synergistic remedy.
In vivo therapeutic efficacy of Au@FePt by means of mixed synergetic remedy
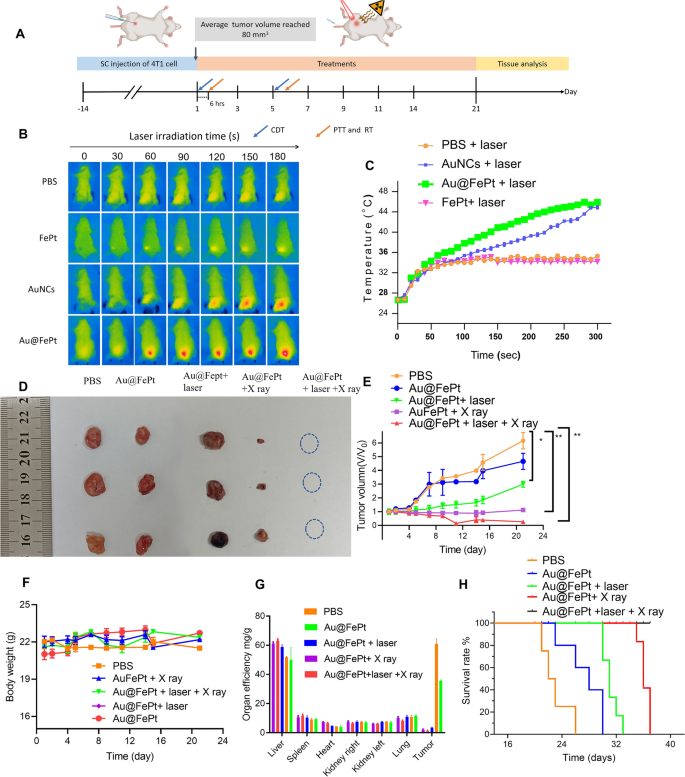
To disclose the anti-tumor efficacy for various remedy teams, the in vivo remedy technique of the orthotopic breast tumor mannequin was noticed in Fig. 7A. For synergistic remedy teams as proven in Fig. 7B, the mice had been additionally injected with Au@FePt (dose: 10 mg/kg) after which irradiated with a NIR laser at 1064 nm with 1.2 W/cm2 for five min at 6 h post-injection, and the IR thermal digital camera recording for the breast most cancers website confirmed a dramatically growing temperature over time. The temperature shortly rose to 44.5 °C after which remained fixed at 45.8 °C for five min after 1064 nm laser publicity for the Au@FePt handled group (Fig. 7B). Subsequently, mice had been injected with AuNCs (10 mg/kg based mostly on the burden of Au focus) and FePt (5 mg/kg based mostly on the burden of Fe focus) and irradiated with laser gentle 6 h after the injection, respectively. The AuNCs handled group additionally strengthened the PTT impact whereas the FePt handled group displayed a reasonable temperature come up to 32 °C. However, after 5 min of laser irradiation, the PBS management group barely modified temperature (from 26 to 32 °C) on the tumor website as proven in (Fig. 7C). The change within the tumor quantity curve and every tumor picture for every group are illustrated in Fig. 7D, E and Extra file 1: Fig. S5, respectively. The numerous tumor accumulation of Au@FePt + laser demonstrated superior anti-tumor efficacy, which was attributed to the AuNCs supply system’s extraordinary heat-generating means. As anticipated, remedy with the Au@FePt NPs + X-ray + laser resulted in a superb therapeutic impact in inhibiting tumor progress, demonstrating the XDT/CDT/PTT mixture remedy impact. As compared, the 4T1 tumors had been injected with an equal quantity of PBS dispersion, and the dimensions of the tumor progress was sooner and uncontrolled.
In vivo XDT/CDT/PTT remedy of the 4T1 tumor-bearing mice after injection of PBS, Au@FePt with and with out laser irradiation or X-ray after 6 h of injection (5 mg/kg, 1.2 W/cm2). A Schedule of therapeutic and evaluation. B Thermal pictures and C corresponding temperature adjustments of tumor website of PBS, Au@FePt with and with out laser irradiation after 6 h post-injection (n = 4). D Images of the tumor systematically administrated after PBS, Au@FePt and Au@Fept + X ray, Au@FePt + laser (1.2 W/cm2) and Au@FePt + laser + X-ray (n = 4). E Relative tumor-growth curve. Insert the pictures of related tumors originating from every group in D. F Physique weight after numerous therapies indicated in 21 days (n = 4). G, H Survival curve and organ effectivity of tumor-bearing mice handled by numerous teams throughout 42 days (n = 4)
Additionally, there was no important physique weight variation after injection of PBS, Au@FePt, Au@FePt + laser, Au@FePt + X-ray and Au@FePt + laser + X-ray below laser irradiation and X-ray remedy as illustrated in Fig. 7F. Determine 7G additionally revealed the change in organ effectivity of every organ within the totally different remedy teams over 21 days. This outcome demonstrated important tumor inhibition in 4T1 tumor-bearing mice injected with Au@FePt NPs with synergistic remedy. Kaplan–Meier survival curves had been used to evaluate therapeutic efficacy. The survival curve was measured each day for as much as 3 weeks as indicated in Fig. 7H. When in comparison with Au@FePt NPs + X-ray or Au@FePt + laser teams, through which most cancers grew at a reasonable pace because of the single impact of gentle PTT with XDT/CDT/PTT in most cancers cells to inhibit tumor angiogenesis, the upper survival charge of the Au@FePt + laser + X-ray handled group could possibly be attributed to synergistic therapies that induce irritation with DNA injury in RCD, thereby downregulating caspase expression within the hypoxic tumor microenvironment through twin induction of ferroptosis and apoptosis [27].
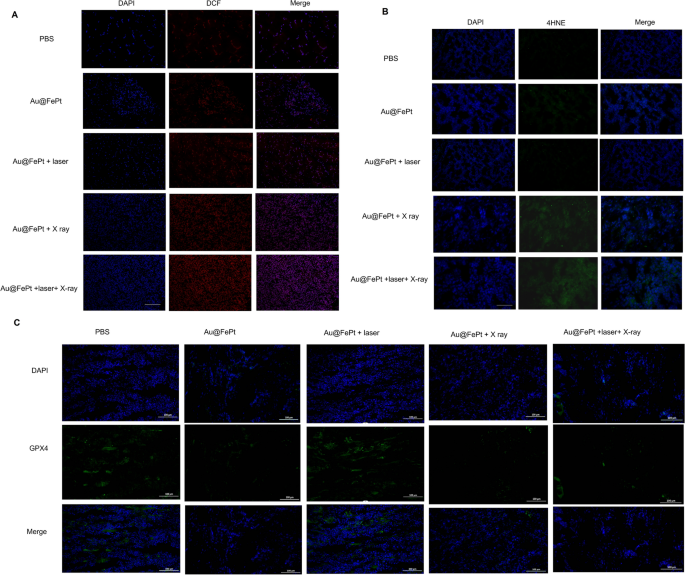
To additional reveal ferroptosis phenomena, an immunohistochemical examination of tumor sections taken from the handled animals was carried out. As proven in Fig. 8A, each Au@FePt-treated teams uncovered to an NIR laser and X ray irradiation had larger ROS ranges. A larger diploma of oxidative stress was noticed within the mice that had been administered Au@FePt + laser + X-ray than in people who had been administered Au@FePt + X ray and Au@FePt + laser. Moreover, as proven in Fig. 8B, the mice handled with Au@FePt + NIR + X-ray produced intratumoral 4-hydroxynonenal (4-HNE), a major class of reactive lipid species, indicating that lipid peroxidation contributes to tumor cell demise [52]. Earlier analysis indicated that below oxidative stress, reminiscent of radiation, the surplus of free radicals results in lipid peroxidation which confirmed the involvement of 4-HNE in tumor cell demise [53].
Intratumoral manufacturing of A ROS and B 4-HNE obtained from tumor tissue slices of mice receiving totally different therapies group. Mice had been sacrificed and tumors had been dissected at 24 h post-irradiation. Scale bar: 50 µm for immunofluorescence pictures and 50 µm for H&E staining pictures. C Intratumoral manufacturing of GPX4 exercise measurement. Knowledge symbolize the imply (± normal deviation, SD) of three impartial experiments
These pathological adjustments had been accompanied by decreased GPX4 exercise, which hyperlinks ferroptosis to the operate of key tumor suppressor pathways (Fig. 8C). Collectively, Au@FePt + laser + X-ray resulted in enough intratumoral GPX4 inactivation in addition to subsequent lipid peroxidation, main to finish tumor eradication. Thus, the usage of Au@FePt + laser + X-ray is a promising therapeutic technique for the ablation of breast most cancers. A sure portion of tumor cell demise through the ferroptosis/apoptosis pathway was noticed for teams of Au@FePt + laser + X-ray, whereas management teams (PBS + laser) revealed no hostile impact on the tumor cell injury (Extra file 1: Fig. S6). Furthermore, an IHC assay with CD31, HIF-1α, and PNAC staining and an immunofluorescent research of tumor tissues had been carried out (Extra file 1: Fig. S8). Obvious shrinkage of cell morphology and breakdown of tumor tissue had been observed within the Au@FePt + laser + X-ray group in contrast with the PBS + laser group, indicating an efficient tumor suppression impact. In keeping with IHC assays with CD31 and PCNA staining, tumor angiogenesis and most cancers cell proliferation had been considerably diminished within the Au@FePt + laser + X-ray group. Moreover, the Au@FePt + laser + X-ray group had a excessive proportion of TUNEL nuclei, implying that apoptosis and ferroptosis might provoke the first pathways of mixed gentle PTT with CDT and XDT, finally resulting in cell demise. PBS as a management group revealed the obvious formation of latest blood vessels. TUNEL staining of Au@FePt + laser + X-ray remedy reveals t extra apoptotic cells than the Au@FePt + laser group and Au@FePt + X ray. The outstanding PTT improved therapeutic final result with the Au@FePt + laser + X-ray together with laser irradiation and X-ray radiation, which holds nice promise as an efficient anti-tumor agent for in vivo tumor remedy.
Biosafety check and blood evaluation
The biosafety check was assessed through H&E staining assay for all nanoprobes within the handled group, and the outcome revealed no apparent acute toxicity (Extra file 1: Fig. S9). We additionally examined the biosafety check through blood evaluation. The blood chemical indices together with alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), plasma uric acid (UA), blood glucose (GLU), and low-density lipoprotein ldl cholesterol (LDL-C) ranges had been measured and revealed no apparent acute toxicity (Extra file 1: Fig. S10).