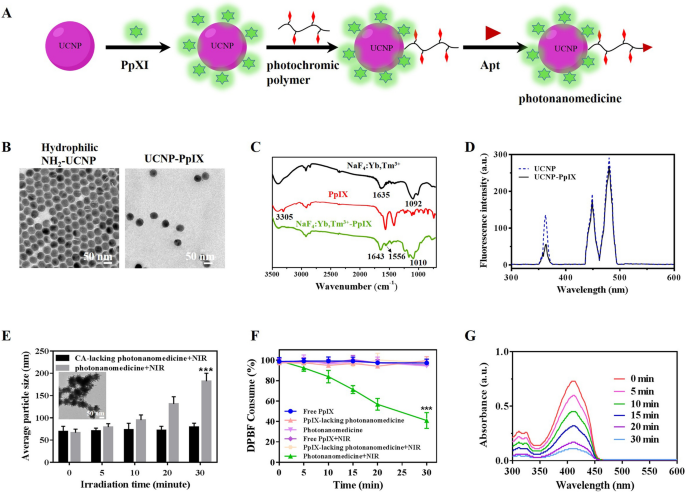
Synthesis and characterization of photonanomedicine
The photonanomedicine was fabricated by way of a number of procedures as indicated in Fig. 1A and Scheme S1. To start with, the amino-modified hydrophilic UCNP was synthesized by a ligand-exchange manner between oleic acid (OA)-coated NaYF4: Yb3+, Tm3+ (OA-UCNP [36, 37], Further file 1: Fig. S1A–D) and 2-aminoethyl dihydrogenphosphate (AEP) in keeping with earlier stories [29, 38]. The profitable alternate was verified by Fourier remodel infrared (FTIR) spectroscopy (Further file 1: Fig. S1E), and the obtained amino-modified hydrophilic UCNP displayed a uniform and spherical morphology (Fig. 1B). Then protoporphyrin IX (PpIX) was conjugated onto hydrophilic UCNP by way of amidation response between the amino teams of UCNP and the carboxyl teams of PpIX to afford UCNP-PpIX. As proven in Fig. 1C, the FTIR outcomes confirmed {that a} new peak at 1010 cm−1 was appeared after conjugating of UCNP with PpIX, which was attributed to the stretching vibration of C-O-N. Apart from, the amide bands C = O stretching vibration was discovered the absorption peak at 1643 cm−1, indicating the profitable synthesize of UCNP-PpIX. The loading content material of PpIX for UCNP was decided by way of the UV-vis methodology and calculated as 2.09%. The TEM picture and dynamic mild scanning (DLS) confirmed that the UCNP-PpIX had a uniform construction with a mean diameter of 47.9 ± 3.1 nm (Fig. 1B and Further file 1: Fig. S1F). As proven in Fig. 1D, the emission depth of UCNP-PpIX at 365 nm decreased when in comparison with that of UCNP, indicating UCNP elicited luminescence may absorbed by PpIX, whose absorption wavelength was at practically 365 nm (Further file 1: Fig. S2). Subsequently, the photochromic polymer was synthesized by way of reversible addition-fragmentation chain switch (RAFT) polymerization adopted by post-polymerization modification (Further file 1: Figs. S3 and S4) [29]. After that, the photochromic polymer was launched onto the UCNP-PpIX by amidation response with remaining amino teams on UCNP. This profitable modification of natural polymer onto inorganic UCNP was verified by TEM. Further file 1: Fig. S5 evidenced a spherical core surrounded by a cloudy-like coating (natural polymer). Lastly, the thiol esters on the floor of ensuing nanoparticles had been diminished to afford thiol-derivatized nanoparticles, which had been then functionalized with the maleimide-modified anti-CD20 aptamer (Apt) to amass the photonanomedicine, whose construction, particularly the conjugation of Apt, was confirmed by agarose gel electrophoresis as reported beforehand (Further file 1: Fig. S6) [29]. The soundness of photonanomedicine and corresponding intermediate nanoparticles in numerous medium, together with phosphate buffer saline (PBS) and serum, had been studied by DLS. As urged in Further file 1: Fig. S7, no aggregates of nanoparticles in all examined medium had been detected for at the least one week, demonstrating the great stability of fabricated photonanomedicines.
Picture-crosslinking and ROS era of photonanomedicine in vitro
We then evaluated the photo-crosslinking capability of photonanomedicine beneath NIR irradiation by way of DLS. Determine 1E confirmed that with the rise of laser time, the scale of photonanomedicine elevated considerably after a 5-min irradiation, and reaching about 300 nm when irradiated for 30 min. This crosslinking capability of photonanomedicine was additional visualized by TEM, during which giant aggregates of photonanomedicine after NIR irradiation had been noticed (inserted picture in Fig. 1E). It’s price noting that, the NIR irradiation didn’t have an effect on the particle dimension of CA-lacking photonanomedicine (Fig. 1E).
Then the ROS era skill of photonanomedicine upon 980 nm NIR laser irradiation was detected by the chemical probe 1, 3-diphenylisobenzofuran (DPBF). As proven in Fig. 1F, G, the absorbance of DPBF decreased considerably with the growing of NIR irradiation time in group of photonanomedicine, and with roughly 60% consumption in 30 min, confirming the environment friendly era of ROS of photonanomedicine. Whereas, the DPBF in different teams didn’t present apparent devour after NIR irradiation.
Synthesis and characterization of photonanomedicine. A Schematic depiction of the fabrication of photonanomedicine. B A consultant TEM picture of hydrophilic amino-functionalized UCNP and PpIX modified UCNP. C Fourier remodel infrared (FTIR) spectra of amino-functionalized UCNP (black curve), PpIX (crimson curve) and UCNP-PpIX (inexperienced curve). D Luminescence emission spectra of UCNP (blue line) and UCNP-PpIX (black line) that had been irradiated by a NIR laser. E Hydrodynamic sizes of CA-lacking photonanomedicine and photonanomedicine upon 980 nm NIR irradiation (2 W/cm2) for varied time durations (insert: a consultant TEM image of photonanomedicine after 980 nm NIR irradiation for 30 min). F Consumption of DPBF over time after completely different therapies. G Absorbance adjustments of DPBF handled with photonanomedicine after 980 nm NIR laser irradiation for varied time durations. Information had been introduced as means ± SD, ***p < 0.001, in contrast with 5 different management group
In vitro synergistic antitumor impact mediated by photonanomedicine
The in vitro therapeutic effectivity of photonanomedicine was assessed, utilizing PpIX-lacking or CA-lacking mono photonanomedicine as management. Firstly, the biocompatibility of those preparations was evaluated on wholesome cells, together with human umbilical vein endothelial cells (HUVEC), human embryonic kidney cells (HEK293) and mouse embryonic fibroblast cells (NIH3T3). No vital discount on cell viability was noticed when the focus of examined preparations reached as much as 1000 µg/mL as displayed in Further file 1: Fig. S8. In the meantime the biocompatibility of NIR irradiation was additionally confirmed, and the outcomes confirmed that NIR irradiation with energy density of two W/cm2 for 30 min (5 min break after 10 min of irradiation) didn’t have an effect on Raji cell viability (Further file 1: Fig. S9). The laser energy was fastened at 2 W/cm2 within the following experiments, until specifically famous.
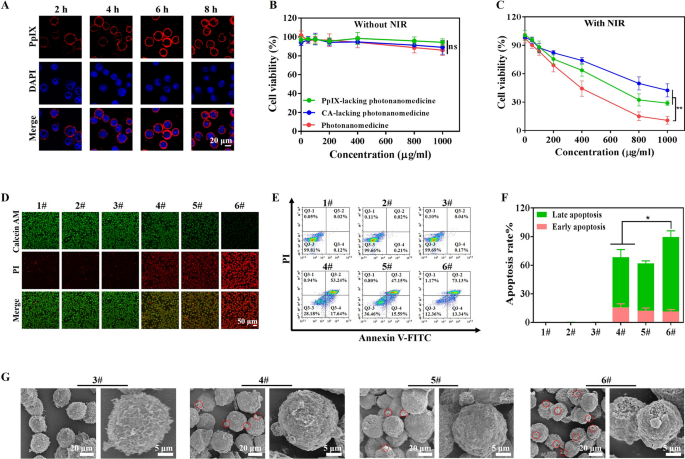
Then we studied the binding effectivity of photonanomedicine onto Raji cells, which had been CD20-overexpressed in comparison with different most cancers cell strains (Further file 1: Fig. S10). As introduced in Further file 1: Fig. S11A, B, the binding capability of photonanomedicine onto Raji cells utilizing CD20 receptor because the anchor was positively correlated with the focus and time. Notably, photonanomedicine may bind onto the membrane of Raji cells and stay on the membrane with out detectable internalization inside ~ 8 h (Fig. 2A), which could as a result of CD20 is a slow-internalized cell-surface receptor. In distinction, when CD20-negative tumor cells, equivalent to Jukart, had been handled with photonanomedicine, they internalized these nanomedicine, on account of missing CD20 anchors (Further file 1: Fig. S12). Moreover, the antitumor effectivity of photonanomedicine plus NIR was investigated by CCK8 assay. As proven in Fig. 2B, C, with out utility of NIR, all of the preparations displayed negligible cytotoxicity in the direction of Raji cells. Whereas upon NIR irradiation, the cell viability was diminished to 27.9% and 40.3% on the focus of 1000 µg/mL, when handled with monotherapy, together with PpIX-lacking (receptor-clustering remedy) or CA-lacking photonanomedicine (PDT). In a stark distinction, photonanomedicine combining receptor-clustering remedy and PDT, displayed the most effective tumor-killing skill with a cell viability of 10.8%, demonstrating synergistic anti-tumor results.
To additional confirm this synergistic effectivity of photonanomedicine, a calcein acetoxymethyl (AM) and propidium iodide (PI) assay was additionally carried out. As depicted in Fig. 2D, most cells had been alive and stained by inexperienced calcein AM, when incubated with preparations with out NIR irradiation. Whereas there have been lifeless cells stained by crimson PI fluorescence when utilized with NIR irradiation, and most lifeless cells had been noticed in photonanomedicine handled group, which was in keeping with the CCK8 assay. Furthermore, Raji cell apoptosis induced by completely different therapies was detected by circulate cytometry. As displayed in Fig. 2E, F, photonanomedicine may induce highest cell apoptosis ration (~ 86.5%), in comparison with ~ 70.9% of PpIX-lacking photonanomedicine or ~ 62.7% of CA-lacking photonanomedicine. This superior anti-tumor capability of photonanomedicine was additional visualized by SEM. As evidenced in Fig. 2G and Further file 1: Fig. S13, cells handled with photonanomedicine plus NIR confirmed the severest membrane injury, and in addition generated essentially the most apoptotic our bodies, in comparison with that of corresponding monotherapy.
In vitro antitumor efficacy research. A Confocal microscopy pictures of Raji cells after publicity to photonanomedicine for varied time durations. B, C In vitro cytotoxicity of Raji cells handled with PpIX-lacking photonanomedicine, CA-lacking photonanomedicine or photonanomedicine with or with out NIR irradiation. D Dwell&lifeless staining fluorescence pictures of Raji cells after varied therapies with or with out NIR irradiation. E Cell apoptosis was evaluated after completely different therapies by circulate cytometry. F Apoptosis charge of Raji cells was calculated primarily based on half E. G SEM evaluation of Raji cells apoptosis after varied therapies. 1#: Management; 2#: NIR; 3#: Photonanomedicine; 4#: PpIX-lacking photnanomedicine + NIR; 5#: CA-lacking photonanomedicine + NIR; 6#: Photonanomedicine + NIR. Information had been introduced as means ± SD, ns means no vital distinction, *p < 0.05, **p < 0.01
Photonanomedicine induced clustering of cell-surface receptor and PDT in vitro
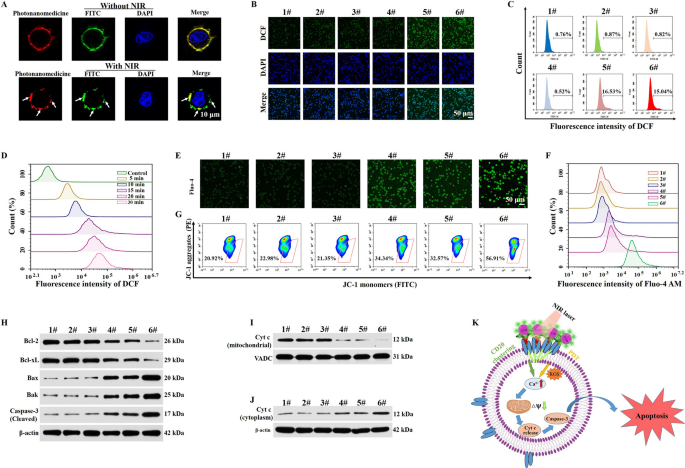
We investigated the anti-tumor mechanism of photonanomedicine. First, the distribution of CD20 receptors on cell floor with or with out NIR irradiation was studied. As proven in Fig. 3A, after an incubation with photonanomedicine, CD20 receptors (inexperienced, stained by FITC-labeled anti-CD20 antibody) in addition to photonanomedicine (crimson) distributed evenly on cell floor. Whereas when the NIR was utilized, CD20 microclusters had been shaped with the crosslinking of photonanomedicine, as urged by the co-localization between inexperienced and crimson fluorescent alerts.
Then we explored whether or not photonanomedicine may exert oxygen to provide ROS upon NIR irradiation, utilizing DCFH-DA probe to detect the intracellular ROS stage. The outcomes urged that the NIR irradiation alone had no impression on the manufacturing of ROS and comparable behaviors had been additionally found for the cells incubated with PpIX-lacking photonanomedicine beneath NIR irradiation. Nevertheless, the fluorescence depth was considerably elevated when the cells had been handled with CA-lacking photonanomedicine or photonanomedicine supplemented with NIR irradiation for five min, indicating that laser irradiation led to considerable ROS era in cells (Fig. 3B, C and Further file 1: Fig. S14). Of word, as soon as the laser irradiation time was prolonged from 5 to 30 min, the fluorescence depth was additionally steadily enhanced, demonstrating that ROS era had a time-dependent method which in keeping with the behaviors in resolution (Fig. 3D and Further file 1: Fig. S15).
In response to the earlier stories and our group’s analysis [29, 39,40,41], the clustering of CD20 receptors at Raji cell-surface led to the aggregation of lipid rafts, adopted by the activation of Src-family protein tyrosine kinases (Src-PTKs) and triggering the inflow of calcium ion, which subsequently resulted within the destruction of mitochondrial membrane potential, activation of caspase-3, and thus the apoptosis. Equally, the produced ROS by way of PDT may injury the cell membrane after which improve the permeability of calcium ion, inducing the mitochondria calcium overload and additional activated mitochondrial-mediated apoptosis pathway (Fig. 3Ok). To confirm the calcium overload mediated by CD20 cluster and PDT, a Fluo-4 AM probe (inexperienced fluorescence [42]) was utilized to measure the intracellular stage of the calcium ion after varied therapies. As displayed in Fig. 3E, F and Further file 1: Fig. S16, the teams of PpIX-lacking photonanomedicine and CA-lacking photonanomedicine upon NIR irradiation confirmed elevated inexperienced fluorescence depth in contrast with the management group, indicating the average inflow of calcium ion. Whereas, cells incubated with photonanomedicine supplemented with NIR irradiation exhibited highest inexperienced fluorescence depth on account of synergy between CD20 cluster and PDT. Additional, mitochondria membrane potential was detected by circulate cytometry and confocal microscopy, utilizing the JC-1 probe [43]. As proven in Fig. 3G and Further file 1: Fig. S17, Raji cells uncovered to photonanomedicine plus NIR irradiation confirmed weakest crimson fluorescence and strongest inexperienced fluorescence depth, indicating a largest lower of mitochondria membrane potential, and thus the best injury of mitochondria.
Subsequently, we measured the downstream alerts of mitochondria injury, particularly the apoptosis-related proteins. As introduced in Fig. 3H and Further file 1: Fig. S18, we discovered photonanomedicine plus NIR handled group exhibited highest expression of pro-apoptotic proteins, equivalent to Bax, Bak and cleaved caspase-3, and lowest expression of anti-apoptotic proteins (Bcl-2 and Bcl-xL) than the monotherapies, i.e. PpIX-lacking or CA-lacking photonanomedicine. Furthermore, we measured the expression stage of cytochrome c in Raji cells after varied therapies. In contrast with 5 different teams, the expression of cytochrome c within the cytoplasm was considerably elevated after photonanomedicine plus NIR remedy, together with a corresponding decline of cytochrome c within the mitochondria-revealing the discharge of cytochrome c from mitochondria into the cytoplasm on account of mitochondria injury (Fig. 3I, J and Further file 1: Fig. S19). Taken collectively, all of the above outcomes demonstrated that the mixed remedy may remarkably improve calcium ion inflow than that of monotherapy, adopted by extreme injury of mitochondria membrane and activation of pro-apoptotic proteins.
Evaluation of antitumor mechanism in vitro. A Fluorescence pictures of the distribution of CD20 receptors on Raji cell floor with or with out NIR irradiation. B Confocal microscopy pictures of ROS era in Raji cells after varied therapies. C Analysis of intracellular ROS manufacturing utilizing the DCFH-DA probe in Raji cells after remedy with completely different preparations by way of circulate cytometry. D Intracellular ROS era was detected in Raji cells handled with photonanomedicine beneath 980 nm NIR irradiation for varied time durations. E Confocal microscopy pictures of Raji cells stained with Fluo-4 AM after completely different therapies. F The circulate cytometry evaluation of intracellular Ca2+ in Raji cells after varied therapies. G The change of mitochondria membrane potential of Raji cells after completely different therapies was objectively measured utilizing circulate cytometry. H Expression of anti-apoptotic proteins (Bcl-2 and Bcl-xL) and pro-apoptotic proteins (Bak, Bax and Cleaved Caspase-3) of Raji cells after varied therapies. I, J Immunoblot evaluation of Cyt c expression in mitochondria and cytoplasm of Raji cells after varied therapies. (Ok) Schematic diagram of the sign occasions concerned within the apoptosis course of. 1#: Management; 2#: NIR; 3#: Photonanomedicine; 4#: PpIX-lacking photnanomedicine + NIR; 5#: CA-lacking photonanomedicine + NIR; 6#: Photonanomedicine + NIR.
Biodistribution of photonanomedicine in tumor-bearing mice after an intravenous injection
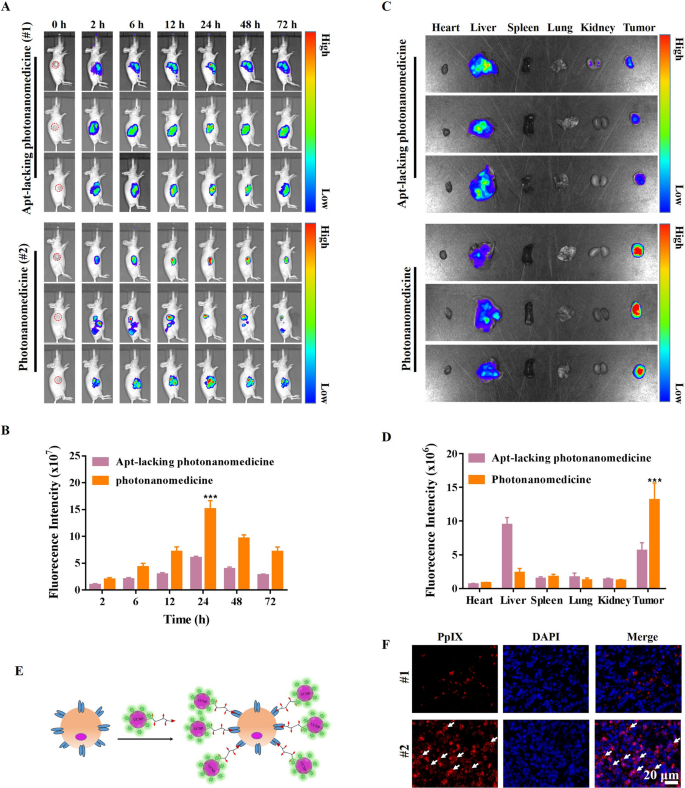
Subsequent, the biodistribution of photonanomedicine after systemic administration was investigated in Raji tumor-bearing mice. As displayed in Fig. 4A, B, photonanomedicine amassed at tumor websites in a time-dependent method, reaching the utmost quantity at 24 h post- i.v. injection. Notably, the amassed photonanomedicine may retain in tumor for at the least 72 h. In distinction, Apt-lacking photonanomedicine displayed the weaker tumor-accumulating capability. Additional the most important organs, together with coronary heart, liver, spleen, lung and kidney, had been collected at 72 h post-i.v. injection, and examined by In Vivo Imaging system. As displayed in Fig. 4C, D, the fluorescent depth of tumors from photonanomedicine handled group was a lot increased than that of Apt-lacking photonanomedicine, additional indicating the improved accumulation of photonanomedicine within the tumor website mediated by the Apt lively focusing on. Furthermore, to discover whether or not the amassed photonanomedicine may goal the cell-surface CD20 of Raji cells in vivo, the tumor slices had been ready and noticed by way of fluorescent microscopy. As anticipated, the outcomes confirmed that photonanomedicine may exactly sure onto tumor cell membrane (white arrows), whereas most of Apt-lacking photonanomedicine was internalized by tumor cells (Fig. 4E, F).
Analysis on focusing on perform of photonanomedicine. A Fluorescence pictures of tumor-bearing mice after intravenous injection of Apt-lacking photonanomedicine and photonanomedicine. The crimson circle marked the tumor website. B The semi-quantitative evaluation of fluorescence depth in tumor websites primarily based on half A. Fluorescence images C and imply fluorescence depth D of tumor tissues and different main organs at 72 h after intravenous injection. E Schematic illustration of photonanomedicine focusing on the Raji cell floor. F The distribution of Apt-lacking photonanomedicine and photonanomedicine in tumor tissues at 72 h post-i.v. injection. Information had been introduced as means ± SD, ***p < 0.001, in contrast with the corresponding management group
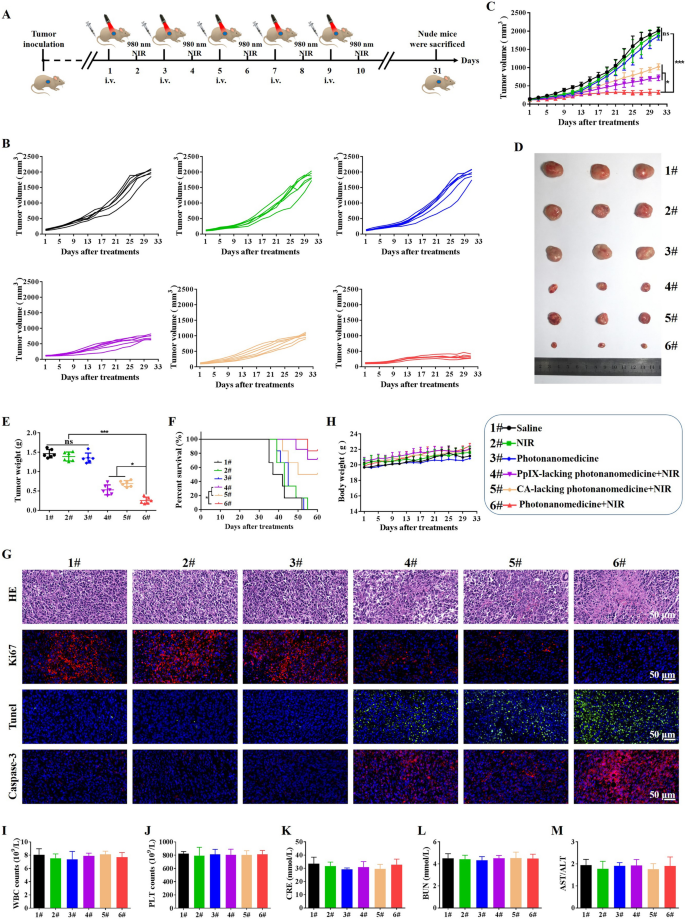
In vivo antitumor efficacy of photonanomedicine
The mixed therapeutic effectivity of photonanomedicine was explored in Raji tumor-bearing mice, and the remedy routine was introduced in Fig. 5A. Tumor-bearing mice had been randomly divided into 6 teams (n = 6), and intravenously receiving completely different brokers as following: (1) Saline; (2) NIR laser; (3) Photonanomedicine; (4) PpIX-lacking photonanomedicine + NIR laser (cell-surface receptors clustering monotherapy); (5) CA-lacking photonanomedicine + NIR laser (PDT monotherapy); (6) Photonanomedicine + NIR laser (mixture remedy). Notably, in situ NIR irradiation was carried out at 24 h post-i.v. injection.
The tumor progress curve for every mouse after receiving corresponding remedy was displayed in Fig. 5B. All of the tumors in mice receiving photonanomedicine plus NIR grew slowest with a tumor inhibition charge of ~ 83.8% (Fig. 5C). In a stark distinction, the monotherapies, i.e. PpIX-lacking photonanomedicine + NIR laser and CA-lacking photonanomedicine + NIR laser, displayed a average tumor inhibition charge of 66.4% and 50.2%, respectively (Fig. 5C). Furthermore, pictures together with weights of tumors collected from mice handled with varied formulations additional verified the synergistic anti-tumor capability of photonanomedicine plus NIR (Fig. 5D, E). Apart from, the survival time of mice handled with photonanomedicine plus NIR was additionally considerably extended in comparison with that of corresponding monotherapies, additional confirming the synergy (Fig. 5F). Moreover, we carried out the H&E evaluation for collected tumor tissues. Most karyorrhexis (fragmentation) and karyolysis (dissolution) had been noticed within the tumor from mice administrated with mixed therapeutic photonanomedicine plus NIR, adopted by the monotherapies, together with PpIX-lacking photonanomedicine + NIR laser and CA-lacking photonanomedicine + NIR laser. Equally, Ki67, Tunel and Caspase-3 evaluation urged photonanomedicine plus NIR induced the most important proportion of cell apoptosis and the least cell proliferation (Fig. 5G).
Subsequent, we studied the biocompatibility of photonanomedicine in vivo. No vital lack of physique weights for mice in all teams had been noticed, suggesting the protection of utilized formulations (Fig. 5H). Furthermore, the H&E staining of main organs, together with coronary heart, liver, spleen, lung and kidney, confirmed that no apparent histopathological abnormalities, degeneration, or lesions, occurred throughout varied therapies (Further file 1: Fig. S20). As well as, we discovered the extent of mobile parts in blood, together with white blood cells (WBC) and platelets (PLT), remained steady after receiving therapies (Fig. 5I, J). In the meantime, the capabilities of kidney (indicated by serum creatinine (CRE), and blood urea nitrogen (BUN)) and liver (indicated by the ration of aspartate transaminase (AST)/alanine aminotransferase (ALT)) weren’t affected after administrated with varied therapies (Fig. 5Ok, L and M).
In vivo antitumor efficacy of photonanomedicine by way of intravenous injection. A Schematic depiction of detailed therapies. B, C Tumor progress curves after completely different therapies. D Digital images of excised tumor tissues from consultant mice after varied therapies. E Tumor weights of mice on the finish time factors. F Survival curves of mice after varied therapies. G Consultant images of tumor sections stained by H&E, Ki67, Tunel and Casepase-3 after varied therapies. H Weight change curves of mice after completely different therapies. I–M Blood biochemical indexes of white blood cells (WBC), platelets (PLT), creatinine (CRE), blood urea nitrogen (BUN) and the ration of aspartate transaminase (AST)/alanine aminotransferase (ALT). Information had been introduced as means ± SD, ns means no vital distinction, *p < 0.05, ***p < 0.001