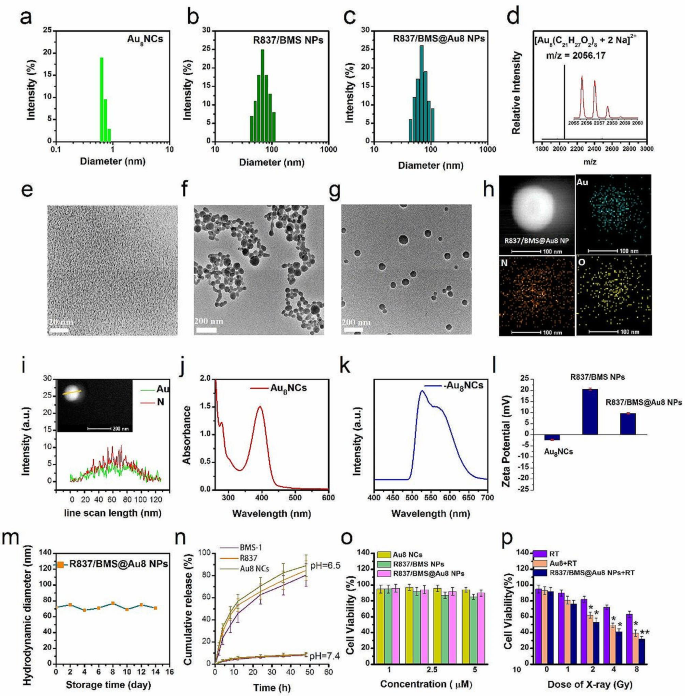
To understand low-dose RT and restrict tumor metastasis, a novel nanocomposite radiosensitizer, R837/BMS@Au8 NP composed of R837, BMS-1, and Au8 clusters was synthesized utilizing nanoprecipitation adopted by electrostatic meeting. Au8NCs have been ready as described [5]. Transmission electron microscopy (TEM) and dynamic mild scattering (DLS) analyses of Au8NCs point out a dimension of < 1 nm (Fig. 1a,e), which is smaller than that reported elsewhere [5, 40]. Au8NCs show narrower dimension distribution and higher monodispersibility. ESI-TOF-MS evaluation confirmed a important peak of doubly-charged Au8NC ion (m/z = 2056.17), similar to [Au8(C21H27O2)8 + 2Na]2+ (Fig. 1d). The picture in insert exhibits the isotopic distribution of the primary peak. The UV–Vis absorption spectrum exhibits that the primary absorption peaks of Au8NCs seem at 225–500 nm (peaks centered at 271, 292, and 388 nm), indicating discrete power ranges (Fig. 1j). The emission spectrum of Au8NCs confirmed intense yellow-green luminescence (Fig. 1okay, Fig. S1), with double emission bands at 519 and 578 nm. Luminescence quantum yield was 67.8%, which can be attributed to higher monodispersibility and stronger quantum results of the Au8NCs. Then, we synthesized R837/BMS NPs with a mass ratio of 1:2 (R837:BMS-1). TEM evaluation confirmed that R837/BMS NPs are spherical nanoparticles with a mean diameter of 68 nm (Fig. 1f). Though R837 and BMS-1 are water insoluble, they comprise hydrophilic teams, akin to carboxyl and amino teams. When an answer of R837 and BMS-1 in acetone was blended with a amount of water, their focus within the answer exceeded vital thermodynamic solubility. They have been in supersaturated states and will co-assemble into an natural composite nanosystem [41, 42].
Properties of nanocomposite radiosensitizer. Dimension distributions of Au8 NCs (a), R837/BMS NPs (b), and R837/BMS@Au8 NPs (c). d Constructive mode ESI-TOF-MS spectrum of Au8NCs. Insets: enlarged portion of the ESI-TOF-MS exhibiting isotopic distribution sample. TEM pictures of Au8NCs (e), R837/BMS NPs (f), and R837/BMS@Au8 NPs (g). h Elemental mapping for the R837/BMS@Au8 NP. The aspect maps present distribution of Au, O, and N. i Elemental line scan exhibits core-shell construction of the R837/BMS@Au8 NP. j,okay UV–Vis and fluorescence spectra of Au8NCs. l Zeta potentials of Au8NCs, R837/BMS NPs, and R837/BMS@Au8 NPs, n = 3. m Storage stability of R837/BMS@Au8 NPs. n Launch profile of Au8NCs, R837, and BMS-1 from R837/BMS@Au8 NPs, n = 3. o Cytotoxicity assay of Au8NCs, R837/BMS NPs, and R837/BMS@Au8 NPs with out radiation, n = 3. p Cytotoxicity assay of Au8NCs and R837/BMS@Au8 NPs with radiation (RT: radiotherapy). Information are introduced as imply ± SD. Statistical significance was decided utilizing the 2 tailed Pupil’s t-test. *P < 0.05, **P < 0.01 compared with RT group, n = 3
Lastly, R837/BMS@Au8 NPs have been synthesized utilizing electrostatic meeting. Au8NCs have a unfavourable ζ potential of − 2.38 mV and R837/BMS NPs have a constructive ζ potential of 20.3 mV (Fig. 1l), leading to that Au8NCs have been assembled on the floor of R837/BMS NPs by electrostatic attraction. Nonetheless, the shaped R837/BMS@Au8 NPs have a constructive ζ potential of 12.2 mV, stabilizing the NPs in aqueous medium by electrostatic repulsion. When Au8NCs have been coated on floor of R837/BMS NPs by electrostatic attraction, the constructive ζ potential on R837/BMS NPs was neutralized, leading to a web constructive ζ potential of 12.2 mV of the shaped R837/BMS@Au8 NPs. This means that the Au8NCs layer was not utterly dense.
TEM evaluation confirmed that R837/BMS@Au8 NPs are spherical, with a mean diameter of 70 nm (Fig. 1g). Aspect mapping of R837/BMS@Au8 NP confirmed the presence of Au in R837/BMS@Au8 NPs (blue-green fluorescent dots point out presence of Au) (Fig. 1h). The sizes of R837/BMS NPs and R837/BMS@Au8 NPs decided by DLS have been according to these decided by TEM (Fig. 1b,c). To confirm the Au8NCs coating on the floor of R837/BMS NP, we carried out an elemental line scan of R837/BMS@Au8 NP (Fig. 1i). These outcomes point out that there’s extra Au aspect than N (in BMS-1 and R837) on the edges of nanoparticles, confirming core-shell construction of the R837/BMS@Au8 NP. The storage experiment shows that R837/BMS@Au8 NPs have been steady in phosphate-buffer saline (PBS) (10 µM) for 14 d (Fig. 1m).
In earlier work, Liu et al. developed a lipid-based nanocarrier doubly loaded with R837 as a toll-like receptor 7 agonist, and caffeine as an adenosine receptor antagonist to restrict the expansion of secondary tumors [43]. In a latest examine, Au/Ag nanorods have been synthesized to inhibit major and distant metastatic tumor progress [44]. In contrast with the 2 nanostructures talked about above, our R837/BMS@Au8 NPs have two benefits: first, their 100% drug-loading capability is helpful for bettering the effectivity of anti-metastatic tumor remedy and overcoming the unwanted effects of carriers; second, Au8NCs with ~ 0.7 nm in dimension are a lot smaller than the kidney filtration threshold (5.5 nm) and might obtain environment friendly renal clearance and cut back liver harm.
Subsequently, our nanocomposite R837/BMS@Au8 NPs confirmed good potential as anti metastatic tumor therapeutic agent as a consequence of its clear composition, good biocompatibility, appropriate granularity, 100% drug loading capability, and environment friendly renal clearance. R837/BMS@Au8 NPs are distinctive amongst reported antitumor nanomedicines used to radioimmunotherapy of tumors. To evaluate the bioavailability of R837/BMS@Au8 NPs, the discharge of BMS-1, R837, and Au8NCs from R837/BMS@Au8 NPs was monitored at pH 6.5, which corresponds to the tumor microenvironment (Fig. 1n). Cumulative launch curves of BMS-1, R837, and Au8NCs from R837/BMS@Au8 NPs have been assessed by UV–Vis and dialysis. After 48 h, BMS-1, R837, and Au8NCs achieved > 80% launch.
For comparability, we carried out a further experiment to guage drug leakage in PBS (pH = 7.4). Primarily based on the outcomes of drug launch outcomes at pH 6.5 and seven.4, we suggest a drug launch mechanism: Though BMS-1, R837, and Au8NCs are water insoluble, they comprise carboxyl, amino, and hydroxyl teams (in ligand of Au8NCs), respectively. Subsequently, they are often protonated to develop into water-soluble at pH 6.5, resulting in the sustained launch of BMS-1, R837, and Au8NCs from the R837/BMS@Au8 NPs. Whereas BMS-1, R837, and Au8NCs could be deprotonated to take care of hydrophobicity at physiological pH, they’re steady and generate a slight launch at pH 7.4 as a result of impact of stirring in the course of the launch course of.
To evaluate the cytotoxicity of Au8NCs, R837/BMS NPs, and R837/BMS@Au8 NPs, the CCK-8 take a look at was used to measure the 4T1 cell response. Au8NCs, R837/BMS NPs, and R837/BMS@Au8 NPs at concentrations < 10 µM displayed > 80% cell viability, and are subsequently, biocompatible at 10 µM (Fig. 1o). The in vitro cytotoxicity of similar focus (10 µM) of Au8NCs and R837/BMS@Au8 NPs irradiated utilizing varied doses of X-rays was measured by evaluating 4T1 cell viability in three cohorts: irradiation solely (purple bars); Au8NCs + irradiation (brown bars), and R837/BMS@Au8 NPs + irradiation (darkish blue bars) (Fig. 1p). Solely RT displayed much less cytotoxicity. Comparatively, as a radiosensitizer, Au8NCs made 4T1 cells extra delicate to radiation, reducing 4T1 cell viability. Moreover, 4T1 cell viability sharply decreased when radioimmunotherapy was primarily based on R837/BMS@Au8 NPs, indicating synergy between RT and immunotherapy.
Because the experimental mannequin used on this examine was devoid of immune cells and the immune adjuvant R837 and small-molecule PD-L1 inhibitor BMS-1 didn’t instantly kill most cancers cells, the cell viability of R837/BMS@Au8 NPs was lowered compared to Au8NCs solely below X-ray irradiation. To clarify this phenomenon, we carried out a drug endocytosis experiment. 4T1 cells have been incubated with R837/BMS@Au8 NPs or Au8NCs with similar focus ( 10 µM) for twenty-four h. Two units of cells have been collected, precipitated, then dispersed in HNO3 answer and digested with H2O2 below boiling circumstances at 200 ℃. ICP-MS was used to find out the Au content material of the samples. The outcomes indicated that the Au content material within the cells containing Au8NC was 63% of that within the cells containing R837/BMS@Au8 NPs (designed 100%). We suggest that as a result of Au8NC is barely ~ 1 nm in dimension, its quantity internalized by cells is lower than that of R837/BMS@Au8 NP at similar time and focus, owing to a R837/BMS@Au8 NP (~ 70 nm) containing many Au8NCs. Subsequently, we conclude that the upper cytotoxicity induced by R837/BMS@Au8 was not associated to the operate of R837 or BMS-1 however fairly to R837/BMS@Au8 NPs, which elevated the endocytic quantity of Au8NCs in cells. The synergistic impact of the three parts of R837/BMS@Au8 not directly kills most cancers cells in vitro. Because the radiation dose elevated, 4T1 cell viability decreased in all three teams. At 1 Gy X-ray, 4T1 cell viability was 43% (< 50%). Hereafter, 1 Gy X-rays are used as radiation supply for in vivo and in vitro antitumor experiments.
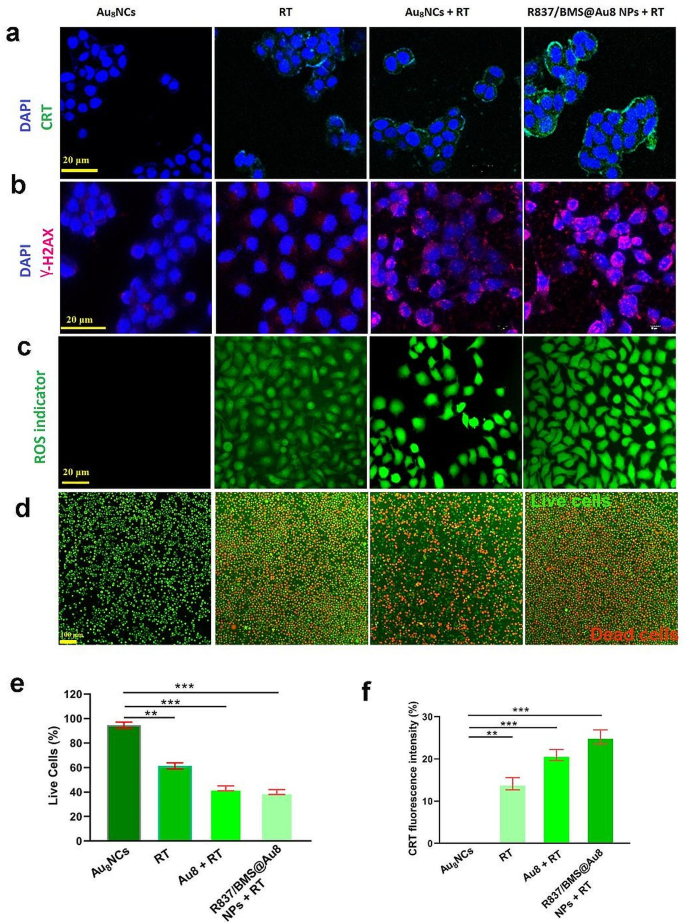
In vitro radiosensitization results. a CLSM pictures of calreticulin expression on 4T1 cells after receiving completely different remedies. b CLSM pictures of γ-H2AX stained 4T1 cells after completely different remedies. c CLSM pictures of ROS era in 4T1 cells after completely different remedies. d Stay/lifeless imaging of 4T1 cells after receiving completely different remedies. Inexperienced: dwell; crimson: lifeless. e The quantity of Stay/lifeless cells after varied remedies. f The quantity of Calreticulin expression on 4T1 cells after varied remedies. Information are introduced as imply ± SD. Statistical significance was decided utilizing the 2 tailed Pupil’s t-test. **P < 0.01, ***P < 0.001 compared with Au8NC group, n = 3
Au nanomaterials have glorious biocompatibility and skill to pay attention native radiation [45]; subsequently, the position of the ready Au8NCs and R837/BMS@Au8 NPs as radiosensitizers have been evaluated in vitro . Briefly, 4T1 cells obtained remedies together with: Au8NCs, RT, Au8NCs + RT, and R837/BMS@Au8 NPs + RT, to guage calreticulin (CRT) expression, reactive oxygen species (ROS) era, DNA breakage, and dwell/lifeless cells.
The anti-immune response throughout RT has attracted nice curiosity not too long ago. RT-induced TAAs have been reported to advertise the antitumor immune response [46]. In most cancers cells, RT induces ICD, which is characterised by excessive CRT expression (“eat me” sign) on the floor of apoptotic most cancers cells. Subsequently, CRT expression was evaluated on the floor of 4T1 cells handled with Au8NCs, 1 Gy RT, Au8NCs + 1 Gy RT, and R837/BMS@Au8 NPs + 1 Gy RT (Fig. 2a,f). The handled cells have been stained with FITC-conjugated anti-CRT antibody and imaged utilizing a confocal microscope. Within the Au8NCs + 1 Gy RT and R837/BMS@Au8 NPs + 1 Gy RT teams, larger CRT expression was seen on the floor of 4T1 most cancers cells than Au8NCs and RT teams. R837/BMS@Au8 induces a better expression of calreticulin (CRT), which isn’t associated to operate of R837 or BMS-1. Nonetheless, as a result of R837/BMS@Au8 NPs enhance the endocytic quantity of Au8NCs in most cancers cells, leading to extra extreme immunogenic cell dying.
Research have proven that utilizing Au NPs as radiosensitizer causes water radiolysis in most cancers cells, producing extra ROS which interacts with biomolecules, resulting in breakage of DNA double strand, cell apoptosis, and so forth. [47, 48]. 4T1 cells handled with Au8NCs or R837/BMS@Au8 NPs have been incubated with DCFH-DA (a probe for ROS) and irradiated with 1 Gy X-rays. ROS produced was detected utilizing a fluorescence microscope (Fig. 2c). Au8 NCs + RT and R837/BMS/Au8 NPs + RT displayed stronger inexperienced fluorescence alerts than RT or Au8NCs.
RT kills most cancers cells via ROS-induced DNA harm. The diploma of DNA harm in 4T1 cells after X-ray irradiation was detected by the immunofluorescence staining of γ-H2AX, which is a marker of DNA harm. γ-H2AX foci have been absent within the nuclei of the Au8NC group and fewer γ-H2AX foci have been current within the nuclei of the RT group (Fig. 2b). In contrast, larger densities of γ-H2AX foci have been present in Au8NCs + RT and R837/BMS@Au8 NPs + RT teams, demonstrating Au8NC-induced DNA breakage. These outcomes are according to these for the ROS era take a look at.
To evaluate the therapeutic impact of our nanocomposite radiosensitizer R837/BMS@Au8 NPs, the cells have been stained with Calcein-AM/PI. Stay and lifeless cell imaging confirmed that 4T1 cells within the PBS group had the very best survival charges (Fig. 2d). Comparatively, 4T1 cells in different teams confirmed completely different levels of apoptosis. Apoptotic cell quantity was highest within the group that obtained radioimmunotherapy primarily based on R837/BMS@Au8 NPs (Fig. 2e).
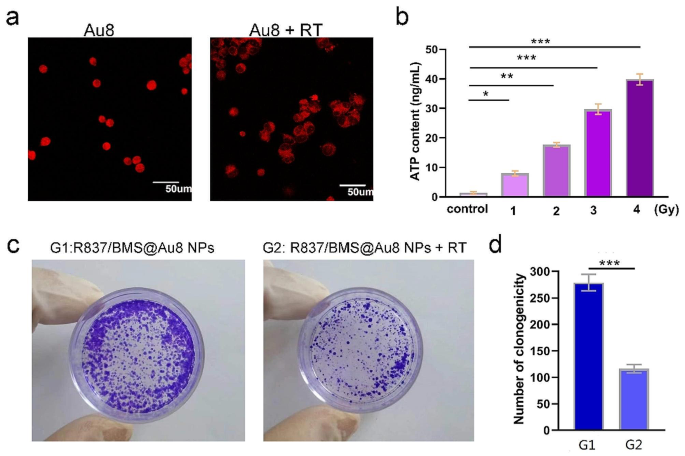
a Immunofluorescence checking was proven and verified the precise localization of HMGB1 earlier than and after irradiation. b Relationship between radiotherapy dose and ATP focus launched by tumor cells. c Consultant pictures of the colony formation assay of 4T1 cells in R837/BMS@Au8 NP and R837/BMS@Au8 NPs + RT teams. d Numbers of clonogenity within the two teams. Information are introduced as imply ± SD. Statistical significance was decided utilizing the 2 tailed Pupil’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001 compared with management or G1 group, n = 3
HMGB1 is a DNA-binding protein launched throughout radiotherapy. Utilizing CLSM, we investigated the cell-specific localization of HMGB1 in 4T1 cells with or with out 1 Gy X-ray remedy. As proven in Fig. 3a, HMGB1 was launched from the nucleus into the cytoplasm. The outcomes steered that in contrast with the non-irradiated teams, the 1 Gy-irradiated cells had an elevated distribution of HMGB1 protein within the cytoplasm.
To confirm that Au8NCs can reply within the proximity of tumor tissues, we assessed the radiation-induced adjustments in ATP focus in tumor cells (Fig. 3b). With rising RT dosage, the focus of ATP within the tumor cell medium progressively elevated, thereby facilitating the response of Au8NCs within the tumor tissue.
To look at the in vitro radiosensitizing results of Au8NCs throughout 14 d, the colony formation assay was carried out (Fig. 3c,d). Within the management group (solely R837/BMS@Au8 NPs), the colonies have been densely packed, indicating that R837/BMS@Au8 NPs exhibit excellent biocompatibility on the experimental focus and had no noticed affect on cell proliferation. In comparison with the R837/BMS@Au8 NP group within the absence of X-ray irradiation, the surviving fractions of the group handled with R837/BMS@Au8 NPs + RT have been clearly decrease.
Thus, R837/BMS@Au8 NPs can successfully induce ROS era, DNA breakage, and CRT expression leading to elevated cell apoptosis and decreased most cancers cell colony formation below X-ray irradiation. R837/BMS@Au8 NPs is an efficient radiosensitizer and could be utilized in subsequent era RT.
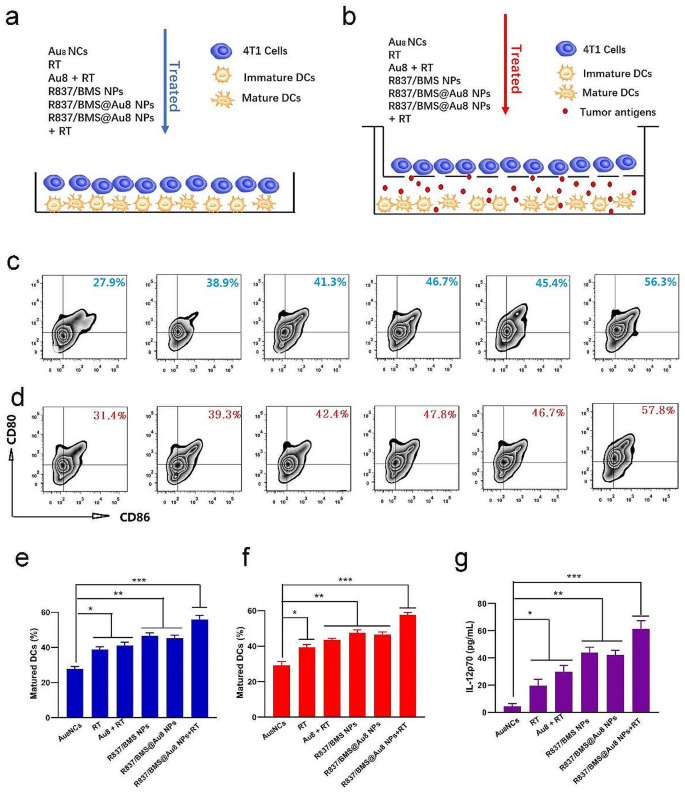
In vitro DC maturation. a, b Schemes displaying the design of two DC maturation experiments in vitro. c, e The quantity of mature DCs after varied remedies in vitro coculture system. d,f The quantity of mature DCs after varied remedies in Transwell system. g IL-12p70 secreted in suspensions of coculture system b. Information are introduced as imply ± SD. Statistical significance was decided utilizing one-way ANOVA take a look at. *P < 0.05, **P < 0.01, ***P < 0.001, n = 3
Immature DCs engulf TAAs and course of them into peptides, that are introduced to the most important histocompatibility advanced (MHC). The MHC–TAA advanced on DC floor is acknowledged by T cell receptors to activate T cells relying on the maturation of DC [49, 50]. This initiates the antitumor immune response. We first investigated in vitro DC maturation stimulated by remedy methods. Immature DCs from the bone marrow have been stimulated with granulocyte macrophage colony stimulating issue after which given varied remedies in a DC/4T1 coculture system. In direct mixing of DCs and 4T1 cells, coculturing of twin cells was adopted by remedies together with PBS, RT, Au8NCs + RT, R837/BMS NPs, R837/BMS@Au8 NPs, and R837/BMS@Au8 NPs + RT (Fig. 4a). Mature DCs have been confirmed by twin staining for CD86 and CD80, that are markers of mature DCs (Fig. 4c,e). The proportion of mature DCs (CD80+ CD86+) within the remedy group with R837/BMS@Au8 NPs + RT was the very best (as much as 56.3%) in contrast with these in different teams. To substantiate this end result, a Transwell system was used with higher and decrease chambers containing 4T1 cells and DCs, respectively, adopted by completely different remedies (Fig. 4b). The frequency of mature DCs (CD80+ CD86+) within the R837/BMS@Au8 NPs + RT remedy group was additionally the very best (57.8%) (Fig. 4d,f). Two units of experimental outcomes proved that probably the most evident maturation of DCs was noticed within the R837/BMS@Au8 NPs + RT group, indicating that radioimmunotherapy primarily based on R837/BMS@Au8 NPs can effectively stimulate DC maturation. Research have reported that tumor cell RT induces TAA era, and R837 acts as an immune adjuvant to stimulate DC maturation. Moreover, the degrees of interleukin 12 (IL-12p70) within the medium of the Transwell system after remedies have been measured through enzyme-linked immunosorbent assay (ELISA). IL-12p70 is a cytokine that’s secreted by DCs. The outcomes confirmed that the altering development of IL-12p70 is much like that of mature DCs in each coculturing modes (Fig. 4g).
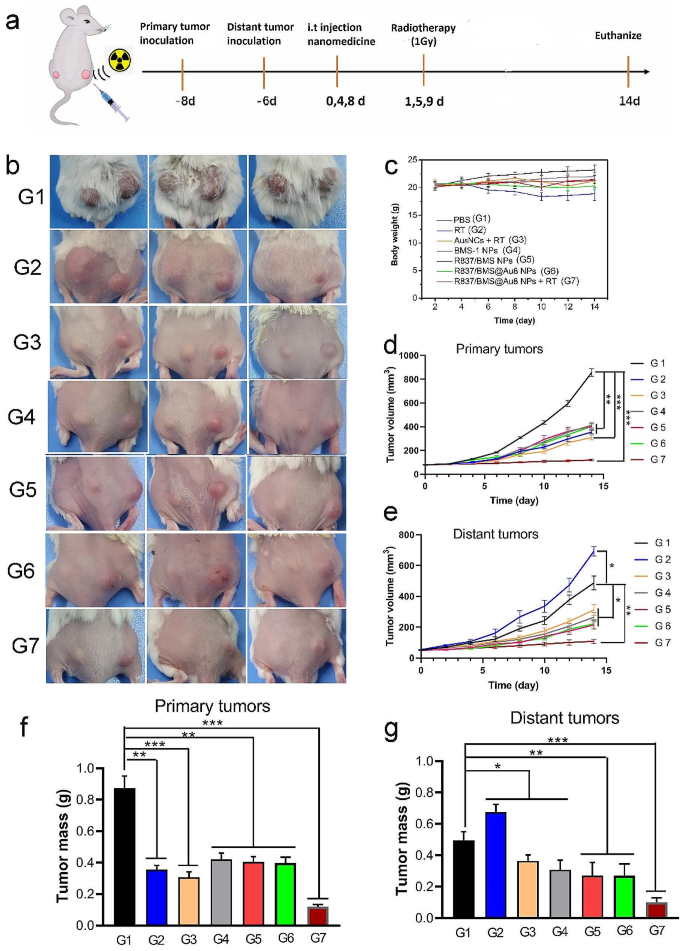
In vivo antitumor take a look at. a Remedy schedule. b Images of euthanized mice after 14 d of remedy. c Adjustments of physique weights of mice throughout tumor remedies. d,e Development curves of major and distant tumor volumes. f,g Weights of major and distant tumors after tumor remedies. Information are introduced as imply ± SD. Statistical significance was decided utilizing one-way ANOVA take a look at. *P < 0.05, **P < 0.01, ***P < 0.001, n = 5
The in vitro results of radioimmunotherapy primarily based on R837/BMS@Au8 NPs + RT impressed us to analyze the consequences of radioimmunotherapy of tumor in vivo. Subsequently, 4T1 cells have been inoculated into the best flanks of the mice as major tumors. After 2 d, distant tumors, as mimic metastasis tumors, have been inoculated into the left flanks of the identical mice (Fig. 5a). Concurrently, 4T1 cells have been injected into the identical mice via the tail vein to kind pulmonary nodular tumors. When major and distant tumors reached roughly 80 and 50 mm3, respectively, the mice have been randomly divided into seven teams (n = 5): (1) PBS; (2) RT solely; (3) Au8NCs + RT; (4) intratumoral (i.t.) injection of BMS-1 NPs; (5) i.t. injection of R837/BMS NPs; (6) i.t. injection of R837/BMS@Au8 NCs; and (7) i.t. injection of R837/BMS@Au8 NCs + RT. Au8 NCs, BMS-1 NPs (Fig S2), R837/BMS NPs, and R837/BMS@Au8 NPs, have been domestically injected into tumors within the corresponding teams at doses of 4 mg/kg for Au8NCs, 1 mg/kg for BMS-1, and 0.5 mg/kg for R837 on day 0, 4, 8 after remedy, respectively. After 24 h, relational major tumors have been radiated with 1 Gy X-ray on day 1, 5, 9 after remedy, respectively. To keep away from cytokine storms, i.t. injection is used for radioimmunotherapy of tumors. Then, tumor dimension on each side was monitored each different day. Major tumors in teams with X-ray radiation, BMS-1 NPs, R837/BMS NPs, and R837/BMS@Au8 NPs confirmed slower progress in contrast with these within the PBS group (Fig. 5b, d). The first tumor remedies in RT and Au8NCs + RT teams have been discovered to be more practical than the above 4 teams together with PBS, BMS-1 NPs, R837/BMS NPs, and R837/BMS@Au8 NPs teams. R837/BMS@Au8 NPs + RT was handiest in inhibiting major tumor progress. Thus, R837/BMS@Au8 NPs might enhance the efficacy of RT by selling absorption of X-rays via Au8NCs, strengthening antitumor immunity via R837, and blocking the PD-1/PD-L1 pathway via BMS-1.
The expansion of distant tumors (as simulated metastatic tumors with out direct remedy) was inhibited probably as a result of activation of antitumor immunity. The distant tumors of mice with major tumors irradiated with 1 Gy displayed fast progress (Fig. 5b,e), as a result of RT alone couldn’t activate the antitumor immune response in mice. The distant tumors of mice with major tumors injected with BMS-1 NPs, R837/BMS NPs or R837/BMS@Au8 NPs had slower progress than these within the RT solely group, demonstrating the significance of R837 in inducing antitumor immunity and BMS-1 in blocking the PD-1/PD-L1 pathway. In RT + Au8NC group, the distant tumors had comparable progress with tumors within the above three teams, indicating that radioimmunotherapy primarily based on Au8NCs induces systemic antitumor immunity. Notably, 3/5 distant tumors with corresponding major tumors handled with injection of R837/BMS@Au8 NPs + RT shrank. Thus, radioimmunotherapy primarily based on R837/BMS@Au8 NPs decreases the dose of X-ray and initiates robust systemic antitumor immunity to restrict tumor metastasis. On the finish of the experiment, all major and distant tumors have been dissected from the mice and weighed. The inhibition charges of major and distant tumors have been 86.6% and 78.2%, respectively (Fig. 5f,g). Moreover, slight adjustments in mice weight have been noticed throughout remedy (Fig. 5c), suggesting low systemic toxicity of assorted therapies. Moreover, radioimmunotherapy primarily based on R837/BMS@Au8 NPs enhanced the survival time of tumor-bearing mice, in contrast with mice within the different 5 teams. Survival interval of 70% mice within the R837/BMS@Au8 NPs + RT group was > 60 d, whereas the survival time of tumor-bearing mice was 36 d within the PBS group (Fig. 6a). ICP-MS evaluation confirmed that Au8NCs have been eradicated within the urine of tumor-bearing mice inside 9 d.
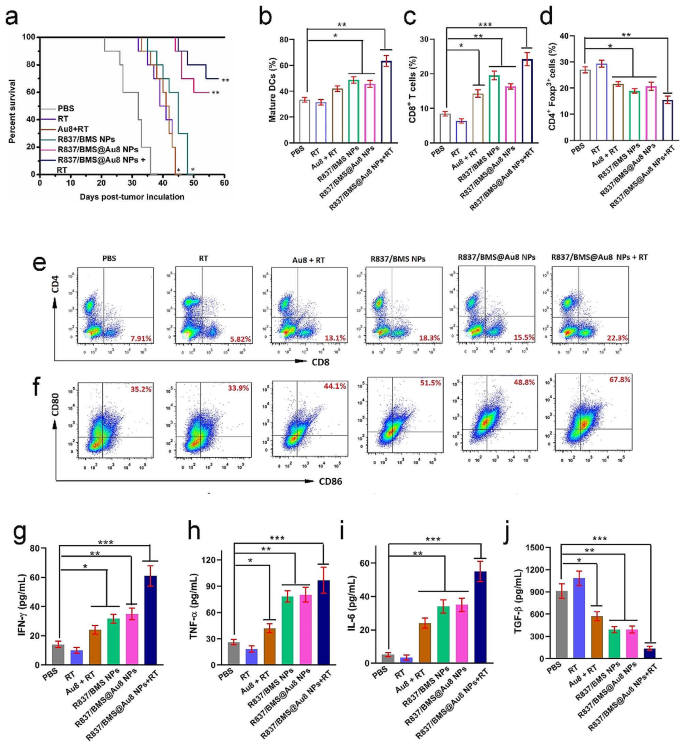
Mechanism of anti metastatic tumor. a Survival charges of mice after varied remedies (n = 10). c,e Infiltration of CD8+cells into distant tumors. d Infiltration of CD4+Foxp3+cells into distant tumors. b,f DC maturation charges from the lymph nodes in bilateral 4T1 tumor mice. g–j Serum IFN-γ, TNF-α, IL-6, and TGF-β ranges. Information are introduced as imply ± SD. Statistical significance was decided utilizing one-way ANOVA take a look at. *P < 0.05, **P < 0.01, ***P < 0.001, n = 3
We then examined the mechanisms of antitumor metastasis throughout radioimmunotherapy primarily based on R837/BMS@Au8 NPs. PD-L1 blockade might stop immune evasion of most cancers cells to fight tumors by reactivating CTLs and inhibiting immune-suppressive Tregs [39]. CTLs (CD3+CD4−CD8+) instantly kill most cancers cells by releasing cytotoxins, akin to IFN-γ, granzymes, granulysin, and perforin [51, 52]. Distant tumors have been faraway from 4T1 tumor-bearing mice. CD8+ CTLs (CD3+CD4−CD8+) have been remoted for analyzing their percentages in several remedy teams by circulation cytometry on day 6 after remedy. In comparison with the management teams (7.91% for PBS group and 5.82% for RT group), Au8 + RT, R837/BMS NP, and R837/BMS@Au8 NP teams had 13.1%, 18.3%, and 15.5% of CD8+ T cells, respectively (Fig. 6c,e). Notably, for the R837/BMS@Au8 NPs + RT group, the frequency of CD8+ CTLs was the very best at 23.3%. Conversely, the share of Tregs (CD4+Foxp3+ T cells) within the R837/BMS@Au8 NPs + RT group was the bottom in distant tumors (Fig. 6d).
On day 5 after remedy, we examined in vivo DC maturation in inguinal lymph nodes, and evaluated ranges of interferon-γ (IFN-γ), tumor necrosis issue α (TNF-α), IL-6, and TGF-β by ELISA in mouse sera. The frequency of mature DCs (CD11c+CD80+CD86+) was highest within the R837/BMS@Au8 + RT group (67.8%) in contrast with PBS, RT, Au8NCs + RT, R837/BMS NPs, R837/BMS@Au8 NPs teams (Fig. 6b,f). We measured the degrees of IFN-γ, TNF-α, IL-6, and TGF-β within the sera of mice from completely different remedy teams on day 4 after remedy. Cytokines are necessary markers of innate and adaptive immunity. IL-6 is proinflammatory; IFN-γ and TNF-α are key markers of mobile immunity and play necessary roles in tumor immunotherapy; and TGF-β is immunosuppressive. Ranges of TNF-α, IL-6, and IFN-γ have been upregulated, whereas that of TGF-β was considerably decreased within the R837/BMS@Au8 NPs + RT group (Fig. 6g–j). Taken collectively, radioimmunotherapy primarily based on R837/BMS@Au8 NPs strengthened in vivo antitumor immunity as a result of adjuvant operate of R837, TAA launch after improved RT by Au8NCs, and blockade of PD-L1 operate by BMS-1.
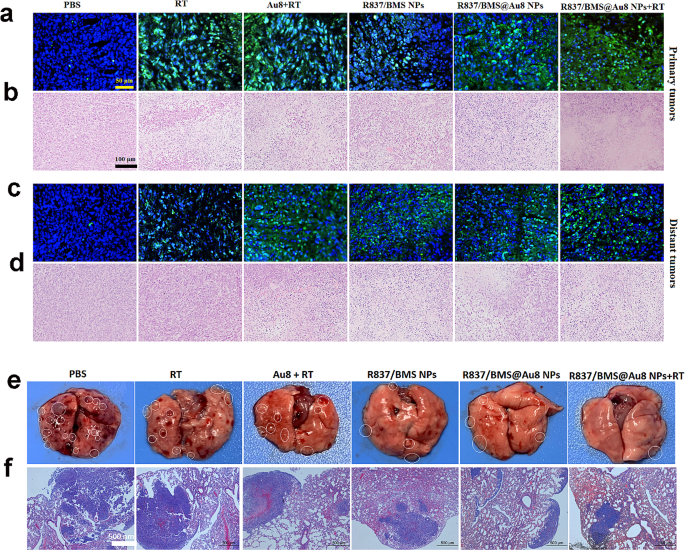
To guage 4T1 tumor cell harm and apoptosis in vivo, sections of major and distant tumors from completely different therapeutic teams have been stained with hematoxylin and eosin (H&E). Totally different levels of mobile harm have been seen in RT, Au8 + RT, R837/BMS NP, and R837/BMS@Au8 NP teams. Probably the most severe harm (cell shrinkage and chromatin condensation) was noticed within the R837/BMS@Au8 NPs + RT group (Fig. 7b). The terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) take a look at was used to detect apoptotic cells. RT, Au8 + RT, R837/BMS NP, and R837/BMS@Au8 NP teams confirmed evident cell apoptosis, whereas the R837/BMS@Au8 NPs + RT group displayed probably the most evident apoptotic cells (Fig. 7a). Photographs for H&E staining and TUNEL confirmed related evaluation outcomes for untreated distant tumor sections (Fig. 7c,d).
To evaluate the impact of remedies limiting the formation of metastatic tumor nodules after tail vein injection of 4T1 cells throughout remedy, the lung was remoted from all teams on the finish of the in vivo antitumor experiment. Many metastatic tumor nodules have been discovered on the floor of the lung in PBS, RT, and Au8NCs + RT teams. In contrast, fewer nodules have been current in teams R837/BMS NP and R837/BMS@Au8 NP. In group R837/BMS@Au8 NPs + RT, just one metastatic nodule was discovered within the lung (Fig. 7e). Lungs with metastatic nodules in several remedy teams have been sectioned and stained with H&E to look at the metastatic space (Fig. 7f). The development of change in tumor nodule quantity correlated with development of change in lung metastasis space. The 2 units of outcomes confirmed that RT utilizing R837/BMS@Au8 NPs as radiosensitizer might successfully restrict lung tumor nodules, probably as a consequence of elevated antitumor immune response and lowered X-ray dose.
The systemic toxicity of nanomedicines is a key index for his or her scientific transformation. H&E staining was carried out on the finish of the antitumor experiment to guage systemic toxicities of the radiosensitizer R837/BMS@Au8 NPs in vivo. Histological evaluation revealed no harm to the guts, liver, spleen, lung, and kidney of mice in all remedy teams (Fig. S3).