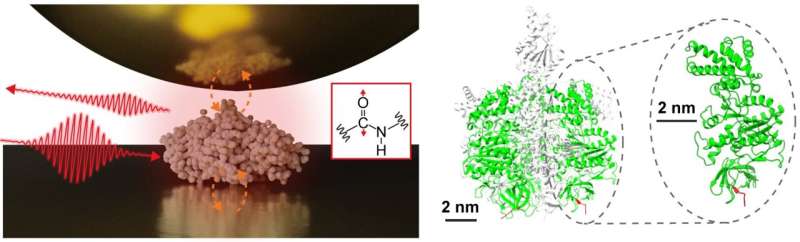
An interdisciplinary analysis staff, led by Assistant Prof. Jun Nishida and Affiliate Prof. Takashi Kumagai on the Institute for Molecular Science, has efficiently noticed vibrational spectra of single proteins, consisting of roughly 500 amino acid residues, utilizing superior measurement methods based mostly on near-field optical microscopy. This technique makes use of mild confined on the nanometer scale, permitting for the detailed evaluation of extraordinarily small samples, which was beforehand difficult with standard infrared spectroscopy.
The research is revealed within the journal Nano Letters.
Standard infrared spectroscopy has been extensively used for the structural and chemical evaluation of assorted supplies as it might probably measure vibrational spectra, sometimes called the “molecular fingerprints.”
The brand new achievement represents a significant development in direction of technological improvements reminiscent of ultra-sensitive and super-resolution infrared imaging, in addition to single-molecule vibrational spectroscopy.
The speedy growth of nanotechnology lately has led to rising demand for ultra-high sensitivity and super-resolution infrared imaging. Nevertheless, standard infrared spectroscopy is restricted in measuring extraordinarily small samples or attaining nanometer-scale spatial decision. For instance, even infrared microspectroscopy with good sensitivity requires over 1,000,000 proteins for acquiring an infrared spectrum, rendering it not possible to measure only a single protein.
Of their research, the analysis staff remoted a single protein, a sub-unit comprising a protein complicated referred to as F1-ATPase, on a gold substrate and carried out near-field infrared spectroscopy measurements in an ambient atmosphere.
They efficiently acquired the infrared vibrational spectrum of a single protein, representing a significant advance that will result in characterizing native structural organizations of particular person proteins. Such info is especially essential for understanding the delicate features of protein complexes and membrane proteins, providing deeper insights into their mechanisms and interactions.
Moreover, they’ve developed a brand new theoretical framework describing the nanoscale interactions between the infrared close to area and protein.
Primarily based on the speculation, the staff was in a position to quantitatively reproduce the experimental vibrational spectra that they noticed. These outcomes will likely be invaluable for the chemical evaluation of biomolecules in addition to numerous nanomaterials, paving the best way for a spread of functions of nanoscale infrared spectroscopy.
Extra info:
Jun Nishida et al, Sub-Tip-Radius Close to-Area Interactions in Nano-FTIR Vibrational Spectroscopy on Single Proteins, Nano Letters (2024). DOI: 10.1021/acs.nanolett.3c03479
Offered by
Nationwide Institutes of Pure Sciences
Quotation:
Analysis staff stories observing vibrational spectra of a single protein with infrared nanospectroscopy (2024, January 10)
retrieved 10 January 2024
from https://phys.org/information/2024-01-team-vibrational-spectra-protein-infrared.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.


