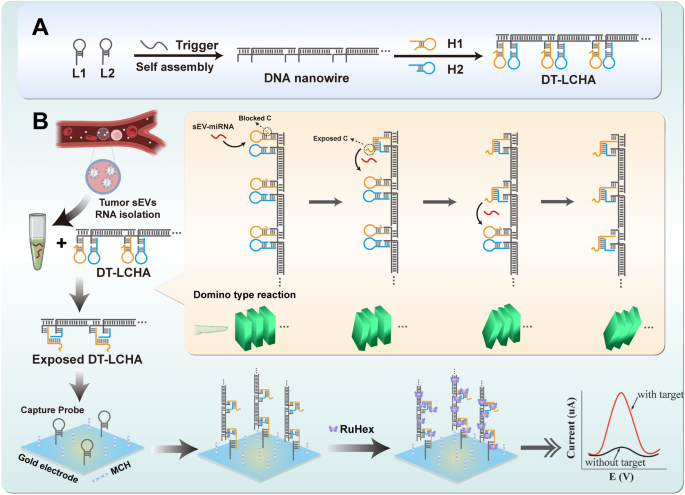
Precept underlying the DT-LCHA platform
The precept underlying the functioning of the DT-LCHA platform is illustrated in Scheme 1. Due to the excessive expression of sEV-miR-1246 in sufferers with gastric tumors, it was used as a mannequin molecule to confirm this idea [36, 37]. Because the design of DT-LCHA in Further file 1: Fig S2, nanowires with sticky ends are fashioned by the hybridization of L1 and L2 within the presence of the set off strand, and the CHA hairpins are loaded onto the sticky ends by complementary base pairing. As soon as the goal sEV-miRNA1246 is added to the response combination, the hairpin H1 opens into straight-stranded H1, which then results in the opening of H2, exposing the blocked c web site and releasing the sEV-miR-1246; the launched sEV-miR-1246 then triggers the following CHA system by fallowing the path of nanowire. On this method, a domino-type triggering of CHA circuits happens, guaranteeing simple activation of quite a few CHA circuits. Subsequent, DT-LCHA may be captured by the pairing of the uncovered c web site with the seize probe. The electrochemical sign molecule RuHex is then electrostatically sure to the DT-LCHA complexes, producing an amplified sign for detecting sEV-miRNA.
Validation and characterization of the DT-LCHA platform
After the CHA system was designed within the method proven in Further file 1: Fig S2, its secondary construction and free vitality had been analyzed utilizing the Nucleic Acid Bundle (NUPACK) software program (Further file 1: Fig S3) [38]. The double-stranded sequence obtained after hybridization of H1 and H2 has a free vitality of − 42.32 kcal/mol, which is decrease than the person free energies of H1 and H2. Subsequently, the H1/H2 hybrid is steady and the CHA response is irreversible. The uncovered c web site of the H1/H2 complicated additional pairs with the seize probe; as a result of the hybrid construction thus fashioned has a decrease free vitality (− 56.20 kcal/mol), it’s steady. These outcomes point out that when CHA is triggered by the goal sEV-miRNA, it will probably kind a steady double-stranded complicated, which is subsequently captured by the seize probe.
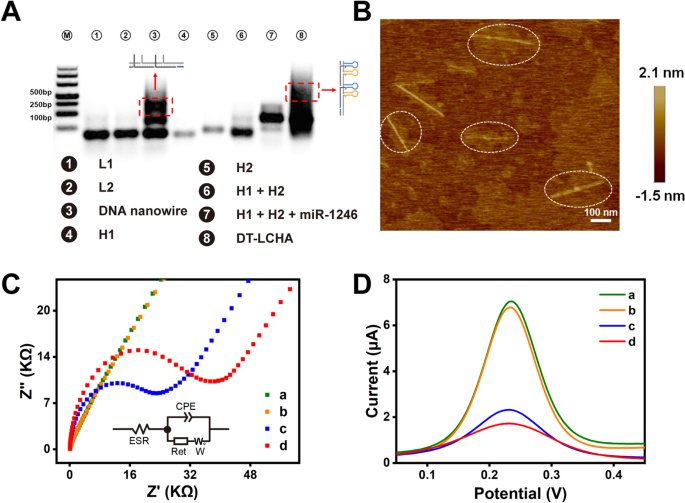
To confirm the meeting of the DT-LCHA complexes, we carried out characterization research utilizing agarose gel electrophoresis. As illustrated in Fig 1A, L1 (lane 1), L2 (lane 2), H1 (lane 4), and H2 (lane 5) had been synthesized as designed, with just one clear band and with none spontaneous response. The band in lane 3 appeared a lot increased, indicating the meeting of L1 and L2 by way of hybridization chain response to kind the double-stranded DNA nanowire. When the nanowire was combined with H1 and H2, a big upward shift of the band was noticed (as seen in lane 8), indicating the profitable meeting of the DT-LCHA complicated. Furthermore, the band in lane 7 confirmed that the CHA system could possibly be efficiently triggered by miRNA-1246, with little background sign. Atomic power microscopy (AFM) was used to additional affirm the meeting and morphology of the DT-LCHA. The AFM photos confirmed a scaffold construction on which linear buildings with a size of ~400 nm had been uniformly dispersed (Fig 1B), which supplied compelling proof of the meeting of the DT-LCHA complicated.
A Agarose gel electrophoresis evaluation of DT-LCHA, lanes from left to proper: Marker; L1; L2; L1 + L2 + Set off; H1; H2; H1 + H2; H1 + H2 + miR-1246; Nanowire + H1 + H2 + miR-1246. B Atom Drive Microscope imaging of DT-LCHA. Characterization of the electrochemical biosensor by EIS (C) and SWV (D) evaluation of naked gold (a); seize probe modification (b); MCH closure (c); DT-LCHA captured by seize probe (d)
The immobilization of the DT-LCHA complicated on the electrode floor was verified utilizing electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), and sq. wave voltammetry (SWV). Within the EIS plots (Fig 1C), the diameter of the semicircle within the plot corresponds to the switch resistance (Ret). The curve for the naked gold electrode is sort of a straight line, indicating good conductivity and low Ret (curve a). After functionalization of the gold electrode floor with seize probes by way of Au–S interplay, the EIS curve confirmed a slight shift, indicating profitable immobilization of the seize probes onto the gold electrode (curve b). In response to the formular of Metal et al. that GDNA = G0 (z/m) NA, the place GDNA is the probe floor density in molecules per cm2, m is the variety of bases in probe DNA, z is the cost of RuHex and NA is the Avogadro’s quantity [39,40,41]. The quantity of seize probe on the electrode floor was calculate as 4.39 × 1012 molecule/cm2. After immobilization of 6-mercaptohexanol (MCH) onto the remaining empty binding websites on the electrode floor, a rise of the Ret was noticed (curve c). This could possibly be because of the enhance within the variety of molecules on the electrode floor. After all of the empty binding websites had been closed utilizing MCH, the immobilization of DT-LCHA complexes onto the electrode floor was carried out; as proven in curve d, addition of 100 nM of the goal molecule, resulted in a big enhance within the Ret. Which means the DT-LCHA was efficiently triggered by the goal molecule on the electrode floor and the resultant complicated was efficiently captured by the seize probe. The kinetic parameters and equal circuit of EIS evaluation had been calculated and extracted in line with the strategies of Anita et al. [42, 43]. The equal circuit can match the EIS plot nicely (Further file 1: Fig S4). The SWV and CV assessments confirmed the identical gradual meeting of the DT-LCHA complicated on the electrode floor, which correlated nicely with the EIS conclusions (Fig 1D and Further file 1: Fig S5). These outcomes absolutely illustrate the profitable meeting and feasibility of the proposed DT-LCHA biosensor.
Triple sign amplification in DT-LCHA
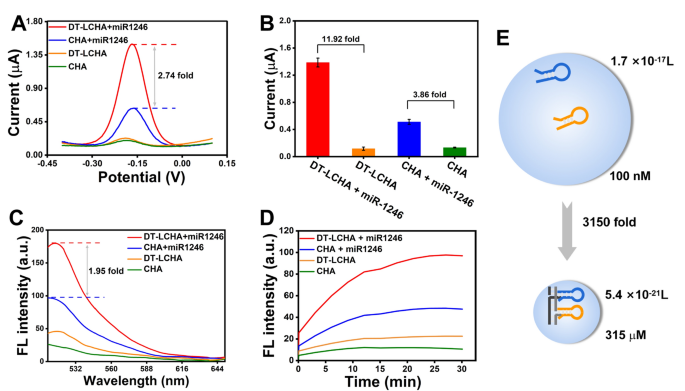
To analyze some great benefits of the DT-LCHA system, the distinction within the electrochemical sign depth between the DT-LCHA and traditional CHA was assessed utilizing the differential pulse voltammetry (DPV) approach. With the addition of miR-1246, the DT-LCHA emitted robust electrochemical alerts with a 2.74-fold sign enhancement in comparison with typical CHA (Fig. 2A). The signal-to-noise ratio was tremendously improved within the DT-LCHA, with a 11.92-fold enchancment as in comparison with a 3.86-fold enchancment in typical CHA (Fig. 2B). These outcomes reveal that the DT-LCHA has an ideal detection vary and facilitates the detection of miRNA targets even when they’re current in a low focus. Fluorescence spectroscopy was used to additional reveal some great benefits of this localized technique. The DNA hairpin H1, modified with the fluorophore FAM and the quencher BHQ1 at reverse websites, can emit fluorescence when triggered by the goal. As proven in Fig. 2C, alerts produced within the DT-LCHA had been 1.95 occasions extra amplified than these in typical CHA. These outcomes reveal the improved amplification of alerts within the DT-LCHA. As well as, kinetic monitoring of fluorescence additional verified the improved amplification of alerts within the DT-LCHA strategy. The fluorescence sign energy in DT-LCHA peaks in about 20 min, and it’s nearly 2 occasions increased than that in typical CHA (Fig. 2D). This proves that the DT-LCHA has good effectivity. Curiously, the electrochemical platform based mostly on the DT-LCHA exhibits increased sign enhancement than the fluorescent platform. That is owing to the truth that within the electrochemical strategy, solely a small variety of CHA circuits want triggering whereas the remaining happen spontaneously as a cascade, and the entire DT-LCHA complicated may be captured by the electrode.
To clarify the sign enhancement impact of localization, we used the collision principle equation V = 1/cN (the place c is the hairpin focus and N represents Avogadro’s fixed). The collision quantity of conventional CHA through the use of collision principle,
$${textual content{V}}_{{{textual content{CHA}}}} = { 1 }/{textual content{ c}}_{{{textual content{CHA}}}} {textual content{N}}$$
the place cCHA characterizes the focus of hairpins 100 nM, and N is Avogadro’s fixed 6.02 × 1023. The amount of conventional CHA was 1.7 × 10–17 L. Then the calculation the quantity of DT-LCHA through the use of sphere calculation components,
$${textual content{V}}_{{{textual content{DT}} – {textual content{LCHA}}}} = { 4 }/{ 3 } occasions , pi {textual content{R}}^{{3}}$$
the place R signify the space between H1 and H2 on the DNA nanowire, the place the space between two hairpins is decreased to 32 base pairs (10.88 nm). The amount of DT-LCHA was 5.4 × 10–21 L. Then calculating the collision focus of localized CHA through the use of collision principle,
$${textual content{c}}_{{{textual content{DT}} – {textual content{LCHA}}}} = { 1 }/{textual content{ V}}_{{{textual content{DT}} – {textual content{LCHA}}}} {textual content{N}}$$
the place V characterizes the quantity of sphere quantity of DT-LCHA. The focus of CHA localized on DNA nanowire was 315 M, which is 3150 occasions increased than that noticed in conventional CHA (Fig. 2E). This exhibits that there’s an enchancment in collision frequency, which leads to increased response pace and effectivity.
Analytical efficiency of the biosensor
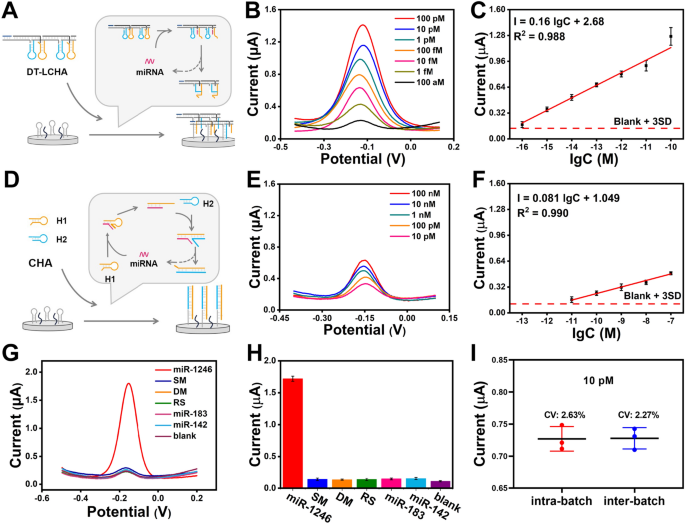
After the optimization of the response temperature and time (Further file 1: Figs S6, S7), the sensitivity of the biosensor was studied utilizing totally different concentrations of sEV-miR-1246. To reveal the superior sensitivity of the developed DT-LCHA biosensor, we performed DPV evaluation on each the DT-LCHA (Fig. 3A) and traditional CHA (Fig. 3D). As illustrated in Fig. 3B, the DT-LCHA exhibited a better sign output than typical CHA (Fig. 3E) on the identical goal focus. Moreover, the DT-LCHA demonstrated a broader linear vary between 100 aM and 100 pM. The linear regression equation of the DT-LCHA was I = 0.16 lgC + 2.68 (R2 = 0.988) (Fig. 3C). Utilizing the clean + 3SD rule, the restrict of detection of the DT-LCHA was discovered to be as little as 24.55 aM, 6213.43 occasions decrease than that of typical CHA (Fig. 3F). These outcomes present complete proof of excessive sensitivity within the DT-LCHA technique. The superior sensitivity of the developed DT-LCHA biosensor could also be associated to the design of triple sign amplification in DT-LCHA. Not solely does the localization impact results in a rise within the variety of CHA hybridization chains triggered per unit time, but in addition the rise within the variety of DNA scaffolds captured on the electrode also can result in a rise in RuHex adsorption, leading to stronger sign output. Moreover, solely a small quantity of CHA must be triggered beneath the situation of extraordinarily low focus of goal miRNA, that’s, DT-LCHA is captured on the electrode, and extra RuHex is adsorbed beneath the motion of nanowire scaffold, ensuing within the enhancement of electrochemical sign. These outcomes present complete proof of excessive sensitivity within the DT-LCHA technique.
Analytical efficiency of the biosensor. A Schematic illustration of the DT-LCHA technique. B DPV responses and (C) corresponding calibration curve of goal miRNA with the focus from 100 aM to 100 pM utilizing DT-LCHA. D Schematic illustration of the custom CHA technique. B DPV responses and (C) corresponding calibration curve of goal miRNA with the focus from 10 pM to 100 nM utilizing DT-LCHA. G DPV sign and (H) DPV outcomes of goal miRNA and 5 interferences. I Intra-batch inter-batch variation of DT-LCHA. The error bars are the usual deviations by three repetitive assays (n = 3)
For ascertaining the specificity of the DT-LCHA-based biosensor, a complete evaluation involving single-base mismatches, double-base mismatches, random sequences, and two homologous sEV-miRNAs (sEV-miR-183 and sEV-miR-142) was performed utilizing DPV assessments. The outcomes, as depicted in Fig. 3G, H, unequivocally reveal an 11-fold increased sign response for absolutely paired targets compared to single-base mutations. This outstanding disparity serves as a testomony to the superb specificity of the proposed platform. The superb specificity of proposed platform because of the correct design of DNA hairpins, particularly the correct distribution of toehold size and double-stranded hybridized half in CHA hairpin. Moreover, the reproducibility of the biosensor was rigorously evaluated by analyzing miRNA at a focus of 10 pM, contemplating each intrabatch and interbatch variability. The obtained information, introduced in Fig. 3I, reveal a variable coefficient of roughly 3%, thereby indicating the platform’s commendable reproducibility. The commendable reproducibility efficiency is attributed to the excessive yield of DT-LCHA ready by six designed DNA strands, which brings unparalleled stability and uniformity.
Compared to various biosensors employed within the detection of sEV-miRNA (Further file 1: Desk S2), the newly devised DT-LCHA-based platform displays superior sensitivity and specificity. Whereas the surface-enhanced Raman scattering (SERS)-based strategy demonstrates heightened sensitivity, the intricate operational necessities and expensive infrastructure related to it might impose restrictions on its scientific feasibility. Conversely, the DT-LCHA-based electrochemical biosensor showcases noteworthy benefits corresponding to economical implementation, fast detection time, and user-friendly operation, thereby exhibiting substantial potential for facilitating point-of-care detection purposes.
Willpower of cell-line-derived sEV-miRNA
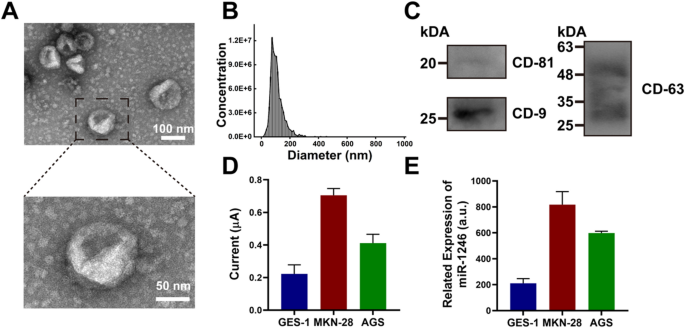
Previous to the willpower of sEV-miRNA, we used the ultracentrifugation technique to extract sEVs from a gastric epithelial cell line (GES-1). The sEVs remoted from the GES-1 cell line had been characterised utilizing normal protocols. The ultrastructure of sEVs beneath a transmission electron microscope revealed a typical disk form with a diameter of 110 nm, which was in line with the outcomes of the nanoparticle monitoring evaluation (NTA) (Fig. 4A, B). The precise marker proteins of sEVs, together with CD-81, CD-9, and CD-63, had been characterised through the use of western blotting (Fig. 4C). The findings reveal that the extracted sEVs meet the definition and exhibit excessive purity.
A TEM photos of sEV extracted from the mannequin cell line GES-1 (higher scale: 100 nm, decrease scale: 50 nm). B NTA evaluation of sEV from GES-1. C Western blot evaluation of sEV marker proteins CD-81, CD-9 and CD-63. D DPV sign of extracted sEV from three mannequin cell traces GES-1, MKN-28 and AGS. E RT-qPCR evaluation of extracted sEV from three mannequin cell traces GES-1, MKN-28 and AGS. The error bars are the usual deviations by three repetitive assays (n = 3)
Subsequently, we chosen a complete of three cell traces—two gastric most cancers cell traces and a standard gastric epithelial cell line. After extracting whole RNA, we used the proposed platform and RT-qPCR to quantify sEV-miR-1246. As proven in Fig. 4D, DPV evaluation confirmed the sEV-miR-1246 expression within the gastric tumor cell traces was increased than that within the regular gastric cell line, with MKN-28 sEVs exhibiting the best miR-1246 expression. The proposed platform exhibited a great correlation with RT-qPCR evaluation for sEV-miR-1246 (Fig. 4E). These outcomes affirm the excessive accuracy of the electrochemical biosensor based mostly on the DT-LCHA.
Medical pattern evaluation on the DT-LCHA platform
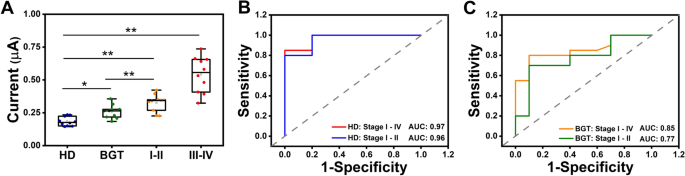
To evaluate the appliance potential of the proposed platform, a cohort of 40 sufferers (n = 40) was recruited, comprising 10 wholesome donors, 10 sufferers with benign gastric tumors, 10 sufferers with gastric most cancers in levels I–II, and 10 sufferers with gastric most cancers in levels III–IV (Further file 1: Desk S3). The DPV alerts of the cohort, depicted in Fig. 5A, revealed a considerably elevated present for sEV-miR-1246 in gastric most cancers sufferers, with an obvious pattern of accelerating ranges because the illness progressed. Moreover, the proposed platform successfully differentiated between the degrees of sEV-miR-1246 in sufferers with gastric most cancers and people in sufferers with benign gastric tumors. To find out the diagnostic efficiency, receiver working attribute (ROC) evaluation was performed, evaluating gastric most cancers sufferers with wholesome donors, yielding a powerful space beneath the curve (AUC) of 0.97 (all levels; CI 0.873–1; sensitivity: 85%; specificity: 100%) and 0.96 (stage I–II; CI 0.883–1; sensitivity: 100%; specificity: 80%), approaching the best worth of 1. Furthermore, diagnostic efficiency was evaluated throughout the cohort of benign gastric tumor sufferers and gastric most cancers sufferers through the use of the proposed platform. ROC evaluation revealed the favorable diagnostic functionality of the proposed platform for gastric most cancers throughout all levels, attaining a sensitivity of 80% and a specificity of 90% (AUC: 0.85; CI 0.688–1). Moreover, it demonstrated promising efficiency in predicting stage I–II gastric most cancers in sufferers and benign gastric tumor in sufferers, yielding a sensitivity of 70% and specificity of 90% (AUC: 0.77; CI 0.553–0.987) (Fig. 5C). The lower off worth of GC sufferers was calculated as 0.251 μA (CI 0.873 to 1; sensitivity of 85%, specificity of 100%) (Further file 1: Fig S8) in line with the Youden index. Collectively, these outcomes present the numerous scientific detection potential of the proposed platform.
Medical pattern evaluation on DT-LCHA platform. A The electrochemical sign for sEV-miR-1246 profiling of Wholesome doners (HD); benign gastric tumor sufferers (BGT); Stage I-II gastric most cancers sufferers (Stage I-II); Stage III-IV gastric most cancers sufferers (Stage III-IV) (*p < 0.05, **p < 0.01; paired t take a look at). B ROC curve between gastric most cancers sufferers (Stage I-IV, Stage I-II) and wholesome donors. C The ROC curve was between gastric most cancers sufferers (Stage I-IV, Stage I-II) and benign gastric tumor sufferers