| Jan 16, 2024 |
|
|
|
(Nanowerk Information) A key chemical response — by which the motion of protons between the floor of an electrode and an electrolyte drives an electrical present — is a important step in lots of vitality applied sciences, together with gas cells and the electrolyzers used to provide hydrogen fuel.
|
|
For the primary time, MIT chemists have mapped out intimately how these proton-coupled electron transfers occur at an electrode floor. Their outcomes may assist researchers design extra environment friendly gas cells, batteries, or different vitality applied sciences.
|
|
“Our advance on this paper was learning and understanding the character of how these electrons and protons couple at a floor website, which is related for catalytic reactions which can be vital within the context of vitality conversion gadgets or catalytic reactions,” says Yogesh Surendranath, a professor of chemistry and chemical engineering at MIT and the senior creator of the examine.
|
|
Amongst their findings, the researchers have been in a position to hint precisely how modifications within the pH of the electrolyte answer surrounding an electrode have an effect on the speed of proton movement and electron move inside the electrode.
|
|
MIT graduate pupil Noah Lewis is the lead creator of the paper, which seems in the present day in Nature Chemistry (“A molecular-level mechanistic framework for interfacial proton-coupled electron switch kinetics”). Ryan Bisbey, a former MIT postdoc; Karl Westendorff, an MIT graduate pupil; and Alexander Soudackov, a analysis scientist at Yale College, are additionally authors of the paper.
|
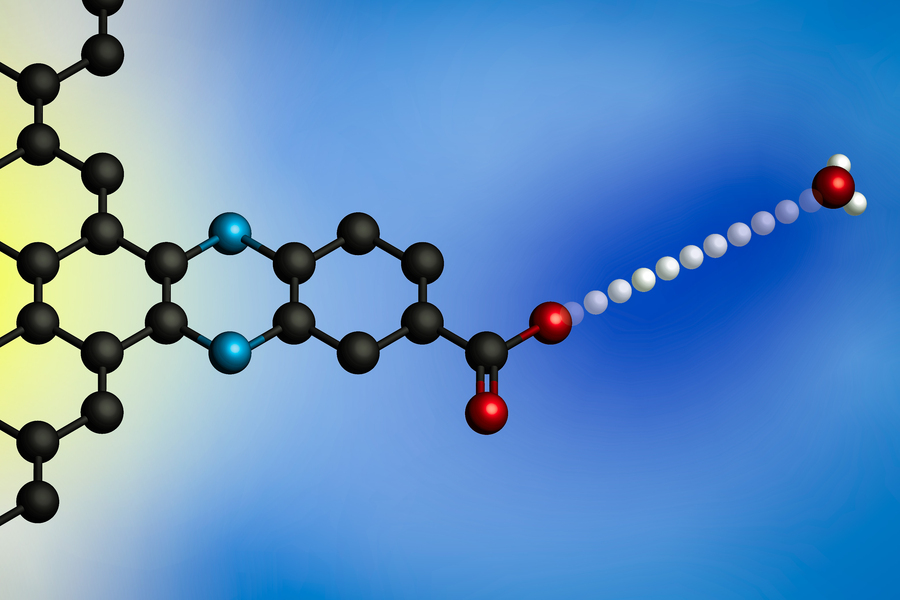 |
| Making use of an electrical potential causes a proton to switch from a hydronium ion (at proper) to an electrode’s floor. Utilizing electrodes with molecularly outlined proton binding websites, MIT researchers developed a common mannequin for these interfacial proton-coupled electron switch reactions. (Picture: Courtesy of the researchers)
|
Passing protons
|
|
Proton-coupled electron switch happens when a molecule, usually water or an acid, transfers a proton to a different molecule or to an electrode floor, which stimulates the proton acceptor to additionally take up an electron. This type of response has been harnessed for a lot of vitality purposes.
|
|
“These proton-coupled electron switch reactions are ubiquitous. They’re usually key steps in catalytic mechanisms, and are significantly vital for vitality conversion processes reminiscent of hydrogen era or gas cell catalysis,” Surendranath says.
|
|
In a hydrogen-generating electrolyzer, this method is used to take away protons from water and add electrons to the protons to kind hydrogen fuel. In a gas cell, electrical energy is generated when protons and electrons are faraway from hydrogen fuel and added to oxygen to kind water.
|
|
Proton-coupled electron switch is frequent in lots of different varieties of chemical reactions, for instance, carbon dioxide discount (the conversion of carbon dioxide into chemical fuels by including electrons and protons). Scientists have discovered an ideal deal about how these reactions happen when the proton acceptors are molecules, as a result of they will exactly management the construction of every molecule and observe how electrons and protons go between them. Nonetheless, when proton-coupled electron switch happens on the floor of an electrode, the method is rather more troublesome to review as a result of electrode surfaces are often very heterogenous, with many alternative websites {that a} proton may probably bind to.
|
|
To beat that impediment, the MIT group developed a technique to design electrode surfaces that provides them rather more exact management over the composition of the electrode floor. Their electrodes include sheets of graphene with natural, ring-containing compounds connected to the floor. On the finish of every of those natural molecules is a negatively charged oxygen ion that may settle for protons from the encircling answer, which causes an electron to move from the circuit into the graphitic floor.
|
|
“We are able to create an electrode that doesn’t include a large range of websites however is a uniform array of a single kind of very well-defined websites that may every bind a proton with the identical affinity,” Surendranath says. “Since we’ve got these very well-defined websites, what this allowed us to do was actually unravel the kinetics of those processes.”
|
|
Utilizing this method, the researchers have been in a position to measure the move {of electrical} present to the electrodes, which allowed them to calculate the speed of proton switch to the oxygen ion on the floor at equilibrium — the state when the charges of proton donation to the floor and proton switch again to answer from the floor are equal. They discovered that the pH of the encircling answer has a major impact on this fee: The very best charges occurred on the excessive ends of the pH scale — pH 0, essentially the most acidic, and pH 14, essentially the most primary.
|
|
To elucidate these outcomes, researchers developed a mannequin based mostly on two potential reactions that may happen on the electrode. Within the first, hydronium ions (H3O+), that are in excessive focus in strongly acidic options, ship protons to the floor oxygen ions, producing water. Within the second, water delivers protons to the floor oxygen ions, producing hydroxide ions (OH–), that are in excessive focus in strongly primary options.
|
|
Nonetheless, the speed at pH 0 is about 4 occasions quicker than the speed at pH 14, partly as a result of hydronium provides up protons at a quicker fee than water.
|
A response to rethink
|
|
The researchers additionally found, to their shock, that the 2 reactions have equal charges not at impartial pH 7, the place hydronium and hydroxide concentrations are equal, however at pH 10, the place the focus of hydroxide ions is 1 million occasions that of hydronium. The mannequin suggests it’s because the ahead response involving proton donation from hydronium or water contributes extra to the general fee than the backward response involving proton elimination by water or hydroxide.
|
|
Current fashions of how these reactions happen at electrode surfaces assume that the ahead and backward reactions contribute equally to the general fee, so the brand new findings counsel that these fashions could must be reconsidered, the researchers say.
|
|
“That’s the default assumption, that the ahead and reverse reactions contribute equally to the response fee,” Surendranath says. “Our discovering is absolutely eye-opening as a result of it signifies that the belief that persons are utilizing to research the whole lot from gas cell catalysis to hydrogen evolution could also be one thing we have to revisit.”
|
|
The researchers at the moment are utilizing their experimental setup to review how including several types of ions to the electrolyte answer surrounding the electrode could pace up or decelerate the speed of proton-coupled electron move.
|
|
“With our system, we all know that our websites are fixed and never affecting one another, so we are able to learn out what the change within the answer is doing to the response on the floor,” Lewis says.
|

