Hydrogen power has emerged as a promising different to fossil fuels, providing a clear and sustainable power supply. Nonetheless, the event of low-cost and environment friendly catalysts for hydrogen evolution response stays a problem.
A analysis crew led by scientists from Metropolis College of Hong Kong (CityU) has just lately developed a novel technique to engineer steady and environment friendly ultrathin nanosheet catalysts by forming Turing buildings with a number of nanotwin crystals. This progressive discovery paves the way in which for enhanced catalyst efficiency for inexperienced hydrogen manufacturing.
The article, titled “Turing structuring with a number of nanotwins to engineer environment friendly and steady catalysts for hydrogen evolution response” is revealed in Nature Communications.
Producing hydrogen by the method of water electrolysis with net-zero carbon emissions is without doubt one of the clear hydrogen manufacturing processes. Whereas low-dimensional nanomaterials with controllable defects or pressure modifications have emerged as energetic electrocatalysts for hydrogen-energy conversion and utilization, the inadequate stability in these supplies attributable to spontaneous structural degradation and pressure rest results in their catalytic efficiency degradation.
To handle this problem, a analysis crew led by Professor Lu Jian, Dean of the School of Engineering at CityU and Director of Hong Kong Department of Nationwide Valuable Metallic Materials Engineering Analysis Middle, has just lately developed a pioneering Turing structuring technique that not solely prompts but additionally stabilizes catalysts by the introduction of high-density nanotwin crystals. This strategy successfully resolves the instability drawback related to low-dimensional supplies in catalytic techniques, enabling environment friendly and long-lasting hydrogen manufacturing.
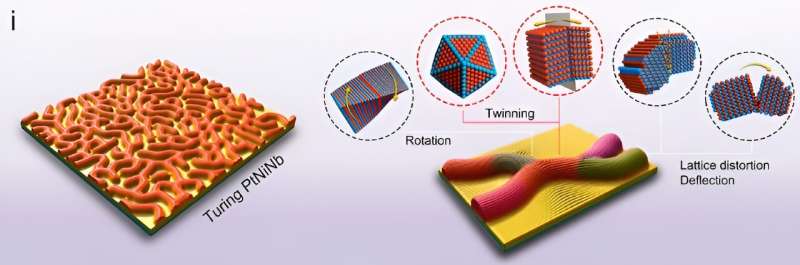
Turing patterns, referred to as spatiotemporal stationary patterns, are broadly noticed in organic and chemical techniques, such because the common floor coloring on seashells. The mechanism of those sample formations is expounded to the reaction-diffusion idea proposed by Alan Turing, a well-known English mathematician considered one of many fathers of contemporary computing, wherein the activator with a smaller diffusion coefficient induces native preferential development.
“In earlier analysis, the fabrication of low-dimensional supplies has primarily centered on structural controls for practical functions, with few concerns on spatiotemporal controls,” stated Professor Lu.
“Nonetheless, the Turing patterns in nanomaterials could also be achieved by the anisotropic development of nanograins of the supplies. Such damaged lattice symmetry has essential crystallographic implications for the expansion of specific configurations, akin to two-dimensional (2D) supplies with twinning and intrinsic damaged symmetry. So we needed to discover the appliance of Turing idea on nanocatalyst development and the relations with crystallographic defects.”
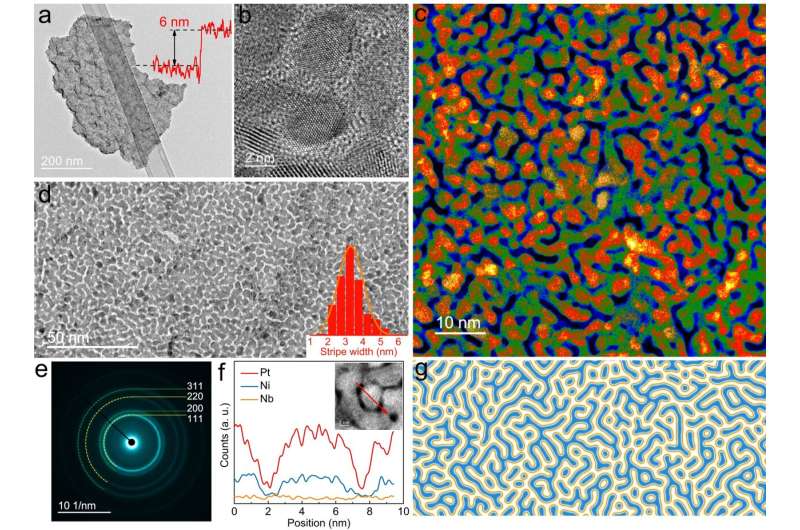
On this analysis, the crew used two-step strategy to create superthin platinum-nickel-niobium (PtNiNb) nanosheets with strips topologically resemble Turing patterns. These Turing buildings on nanosheets have been fashioned by the constrained orientation attachment of nanograins, leading to an intrinsically steady, high-density nanotwin community that acted as structural stabilizers that prevented spontaneous structural degradation and pressure rest.
Furthermore, the Turing patterns generated lattice straining results that scale back the power barrier of water dissociation and optimize the hydrogen adsorption free power for hydrogen evolution response, enhancing the exercise of the catalysts and offering distinctive stability. The floor of the nano-scale Turing construction displays numerous twin interfaces, additionally rendering it an exceptionally well-suited materials for interface-dominated functions, significantly electrochemical catalysis.
Within the experiments, the researchers demonstrated the potential of the newly invented Turing PtNiNb nano-catalyst as a steady hydrogen evolution catalyst with very good effectivity. It achieved will increase in mass exercise and stability index of 23.5 and three.1 instances, respectively, in contrast with business 20% Pt/C. The Turing PtNiNb-based anion-exchange-membrane water electrolyzer with a low platinum (Pt) mass loading of 0.05 mg cm−2 was additionally extraordinarily dependable, because it might obtain 500 hours of stability at 1,000 mAcm−2.
“Our key findings present priceless insights into the activation and stabilization of catalytic supplies with low dimensions. It presents a recent paradigm for enhancing catalyst efficiency,” stated Professor Lu. “The Turing construction optimization technique not solely addresses the difficulty of stability degradation in low-dimensional supplies but additionally serves as a flexible materials optimization strategy relevant to different alloying and catalytic techniques, finally enhancing catalytic efficiency.”
Extra data:
Jialun Gu et al, Turing structuring with a number of nanotwins to engineer environment friendly and steady catalysts for hydrogen evolution response, Nature Communications (2023). DOI: 10.1038/s41467-023-40972-w
Offered by
Metropolis College of Hong Kong
Quotation:
Engineering steady and environment friendly nanosheet catalysts with Turing buildings for hydrogen manufacturing (2024, January 8)
retrieved 8 January 2024
from https://phys.org/information/2024-01-stable-efficient-nanosheet-catalysts-turing.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.


