Twin blockade of LAG3 and TIGIT improves therapy end result of NBTXR3-mediated immunoradiotherapy
To deal with the upregulation of LAG3 and TIGIT induced by the therapy of NBTXR3 + XRT + αPD1, we established a two-tumor mannequin with 344SQR αPD1-resistant lung most cancers in mice, which had been subsequently handled with numerous mixtures of radiation (XRT), XRT enhanced with NBTXR3, αPD1, αLAG3, and αTIGIT (Fig. 1A). In step with our beforehand revealed outcomes [20], irradiation of tumors injected with NBTXR3 and handled with αPD1 produced superior management of tumor progress and longer animal survival time than XRT + αPD1 with out nanoparticle injection (Fig. 1B). The mix of triple checkpoint blockade (αPD1 + αLAG3 + αTIGIT, hereafter abbreviated PLT) within the absence of any radiotherapy (XRT alone or NBTXR3 + XRT) didn’t obtain vital management of both the first or the secondary tumors. As well as, the co-blockade of LAG3 and TIGIT didn’t improve therapy outcomes of XRT + αPD1 with out NBTXR3. Nonetheless, including NBTXR3 + XRT + PLT led to considerably slower progress of each the first and the secondary tumors in addition to prolonged survival. The median survival occasions of every group, in days, had been as follows: management (16), XRT + αPD1 (21), PLT (17), XRT + PLT (24), NBTXR3 + XRT + αPD1 (25), and NBTXR3 + XRT + PLT (35) (Fig. 1B, C). NBTXR3 + XRT + PLT markedly slowed the tumor progress in many of the handled mice, and in 25% (2 out of 8) of the mice that obtained NBTXR3 + XRT + PLT, the tumors had been utterly eradicated (Further file 1: Fig. S2). In distinction, no mice from any of the opposite therapy teams survived the complete assay. Though NBTXR3 + XRT + αPD1 was efficient in delaying the expansion of each the first and secondary tumors in many of the mice, it was finally unable to cease tumor progress in any of them.
Having established the prevalence of NBTXR3 + XRT + PLT, we subsequent sought to guage the advantage of including both αLAG3 or αTIGIT individually to NBTXR3 + XRT + αPD1. Both αTIGIT or αLAG3 was capable of considerably enhance management of each the first and the secondary tumors, and no considerably completely different therapy efficacy was noticed between NBTXR3 + XRT + αPD1 + αLAG3 and NBTXR3 + XRT + αPD1 + αTIGIT by way of tumor progress or survival (Fig. 1C). Nonetheless, neither of those two mixture therapies achieved remission of the tumors. In distinction, on this specific survival assay, therapy with NBTXR3 + XRT + PLT resulted in 3 of the 8 mice (37.5%) being completely cured from their tumors (Fig. 1C).
Beforehand, we noticed that improved management of main and secondary tumors was accompanied by fewer lung metastases [20]. To substantiate this lead to our current research, we counted the variety of metastatic lesions within the lungs of our mice on day 21. In line with our prior observations, lung metastasis corresponded sharply with management of the first and secondary tumors (Further file 1: Fig. S3). Each therapy group paired NBTXR3 with any mixture of CPIs considerably diminished the variety of spontaneous lung metastases in comparison with the management. The addition of both αLAG3 or αTIGIT to NBTXR3 + XRT + αPD1 resulted in considerably fewer lung metastases, and the addition of each in live performance achieved the bottom numbers of metastases of any therapy group.
Lastly, we monitored the physique weight of the mice implanted with 344SQR tumors adopted by therapy with NBTXR3 + XRT + PLT and people untreated naïve mice, no vital distinction in physique weight was noticed between the 2 teams (Further file 1: Fig. S4).
The therapy efficacy of NBTXR3 + XRT + CPIs is closely depending on immune cells
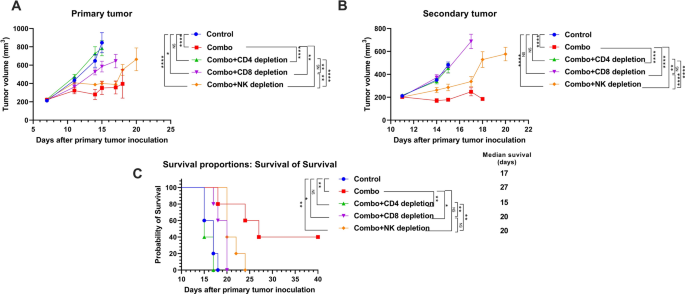
The abscopal impact is considered mediated by the immune response [22]. To elucidate if the therapy advantages we noticed in our NBTXR3 + XRT + PLT therapy group had been certainly immune-mediated and, in that case, what populations of immune cells had been concerned within the antitumor exercise, we depleted CD4+ T cells, CD8+ T cells, and NK cells with antibodies from mice on this therapy group. The depletion of CD4+ T cells, CD8+ T cells, and NK cells all detrimentally impacted the therapy efficacy of the NBTXR3 + XRT + PLT remedy, however at completely different ranges (Fig. 2). CD4+ T cell depletion utterly ablated the tumor management efficacy of the NBTXR3 + XRT + PLT remedy, leading to tumor progress and survival curves just about indistinguishable from these of untreated controls (Fig. 2A–C). Depletion of CD8+ T cells impaired however didn’t completely ablate management of main tumor progress and total survival within the NBTXR3 + XRT + PLT group; like with CD4+ depletion, nevertheless, secondary tumor progress was utterly unrestrained. NK cell depletion had the least impression, with main tumor progress curves solely barely inferior to handled mice who weren’t immunodepleted; the distinction in secondary tumor quantity was extra substantial, however nonetheless the least affected of the immuno-depleted therapy teams. The survival time of NK-depleted mice was barely improved over these of CD8-depleted. In any case, all immunodepletion interventions impaired the therapy efficacy of NBTXR3 + XRT + PLT. Two out of the 5 mice from the non-immunodepleted group survived the complete experiment; not one of the others did. Our outcomes reveal that CD4+ T cells, CD8+ T cells, and NK cells are all important to the efficacy of the NBTXR3 + XRT + PLT therapy, with CD4+ T cells being probably the most indispensable and NK cells the least.
The therapy efficacy of NBTXR3 + XRT + PLT (Combo) closely relies on immune cells. Mice (n = 5) had been handled by the mixture remedy of NBTXR3 + XRT + PLT, as described in Fig. 1. As well as, αCD4 (500 µg), αCD8 (500 µg), and αAsialo GM1 (30 µL) antibodies got by way of intraperitoneal injection on days 5, 7, 9, and 12, and 17 to deplete CD4+ T cells, CD8+ T cells, and NK cells, respectively. Mice had been euthanized when the first or the secondary tumors reached 14 mm in any dimension. A Tumor quantity of the first tumor. B Tumor quantity of the secondary tumor. C Survival charges of the mice. Tumor volumes had been in contrast by two-way evaluation of variance and had been expressed as imply tumor quantity ± commonplace error of the imply ± SEM. Mouse survival charges had been analyzed with the Kaplan–Meier methodology. P < 0.05 was thought of statistically vital. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS, not vital
Twin blockade of LAG3 and TIGIT will increase proliferation of CD4+ and CD8+ T cells
By now, we had established two factors: that mice handled with NBTXR3 + XRT + PLT might management tumor progress and survive in a approach that XRT + PLT handled mice had been unable to match, and that this capacity was dependent upon T cells. Given these two factors, we determined to look at what impact this therapy might need on these immune cell populations. TIGIT and LAG3 are well-known to induce T cell exhaustion. Thus, we reasoned that these two markers’ blockade may alleviate T cell exhaustion.
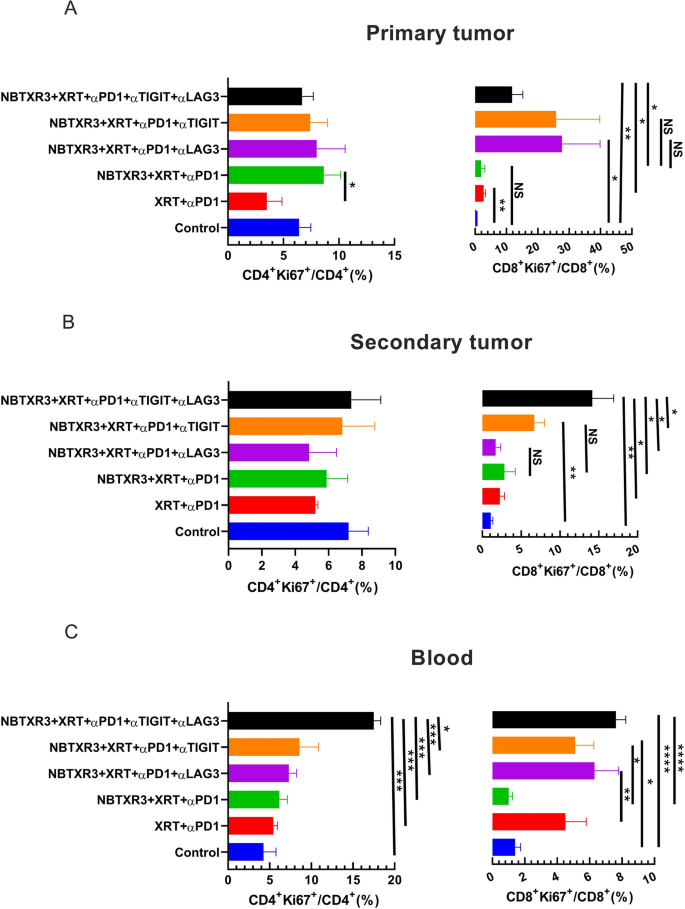
To guage this speculation, we analyzed the proliferation of each CD4+ and CD8+ T cells, as assessed by Ki67 expression, in mice handled with XRT + αPD1, NBTXR3 + XRT + αPD1, NBTXR3 + XRT + αPD1 + αLAG3, NBTXR3 + XRT + αPD1 + αTIGIT, and NBTXR3 + XRT + PLT. No vital variations had been detected within the share of CD4+Ki67+/CD4+ T cells within the main or the secondary tumors between the experimental teams (Fig. 3A, B and Further file 1: Fig. S5A, B, and D). In distinction, proliferating CD8+ T cells elevated within the main tumors of mice handled with αTIGIT and/or αLAG3 (Fig. 3 A and Further file 1: Fig. S5A), a considerably larger share of CD8+Ki67+ T cells was noticed within the secondary tumors in mice handled with both αTIGIT (however not αLAG3), and a a lot higher share was noticed within the secondary tumors of mice handled with NBTXR3 + XRT + PLT (Fig. 3B and Further file 1: Fig. S5B). Within the blood, stream cytometry evaluation present that NBTXR3 + XRT + PLT produced a considerably larger share of Ki67+CD4+ T cells than all different mixture therapies and a considerably larger share of Ki67+CD8+ T cells than the NBTXR3 + XRT + αPD1 group, however not the NBTXR3 + XRT + αPD1 + αLAG3 or NBTXR3 + XRT + αPD1 + αTIGIT group (Fig. 3C and Further file 1: Fig. S5C). These outcomes present that twin blockade of LAG3 and TIGIT, in live performance with NBTXR3-amplified radiation remedy and PD1 blockade, promotes the proliferation of intratumoral proliferation of CD8+ T cells and the systemic proliferation of each CD4+ and CD8+ T cells. As well as, nanostring evaluation of twin blockade of LAG3 and TIGIT didn’t considerably change the variety of CD8+ T, NK, B, and Treg cells within the main or secondary tumors. Nonetheless, the mice handled with NBTXR3 + XRT + PLT exhibited extra CD45+ immune cells within the secondary tumors (Further file 1: Fig. S7).
Twin blockade of TIGIT and LAG3 promotes proliferation of CD4+ and CD8+ T cells. A Percentages of Ki67+CD4+ and Ki67+CD8+ T cells within the main tumors. B Percentages of Ki67+CD4+ and Ki67+CD8+ T cells within the secondary tumors. C Percentages of Ki67+CD4+ and Ki67+CD8+ T cells within the blood. The mice (n = 5) had been handled with numerous mixture therapies, together with XRT + αPD1, NBTXR3 + XRT + αPD1, NBTXR3 + XRT + αPD1 + αLAG3, NBTXR3 + XRT + αPD1 + αTIGIT, and NBTXR3 + XRT + PLT as indicated in Fig. 1 A and had been sacrificed on day 21. The mice which had been inoculated with tumors solely served as management. Immune cells from main tumors, secondary tumors, and blood had been processed and stained with αCD45-APC-Cy7, αCD3-PE-Cy7, αCD4-alexa 700, αCD8-PercpCy5.5, and αKi67-alexa 647. The cells had been run with a Gallios Stream Cytometer (Beckman Coulter) and analyzed with Kaluza software program Model 2.1. The info had been expressed as imply ± SEM and had been analyzed with a two-tailed t check. P < 0.05 was thought of statistically vital. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
NBTXR3, in tandem with triple CPIs, stimulates the activation of immunological genetic applications
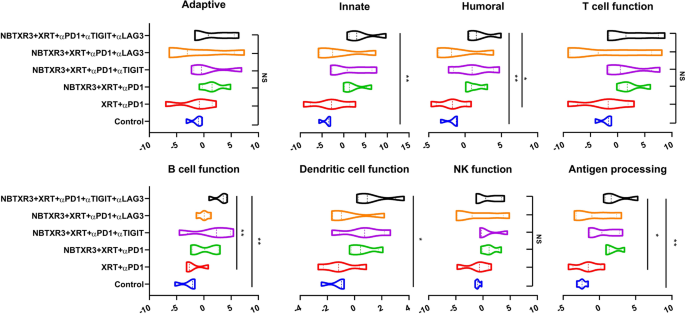
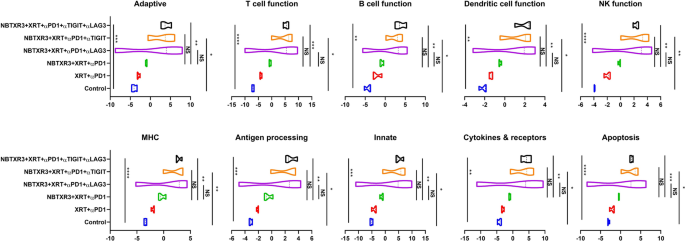
Having delineated the efficacy of those combinatorial therapies in our mouse mannequin, we subsequent sought to parse the genetic responses to every therapy. To this finish, we excised main tumors from the mice and subjected them to finish mobile dissolution, adopted by RNA extraction. We then analyzed the comparative abundance of 770 completely different immune-related genes utilizing the Nanostring PanCancer Immune Profiling Superior Evaluation Module. We noticed vital elevations within the expression ranges of genes concerned in innate immunity, humoral immunity, B cell perform, DC perform, and antigen processing in mice that had been handled with NBTXR3 + XRT + PLT when in comparison with the management (Fig. 4). We additionally noticed elevations in genes concerned in adaptive immunity, T cell perform, and NK cell perform; nevertheless, these will increase weren’t statistically vital. Comparable elevations had been noticed for NBTXR3 + XRT + αPD1 and αLAG3 or NBTXR3 + XRT + αPD1 and αTIGIT, although these weren’t considerably larger than therapy with simply NBTXR3 + XRT + αPD1. Remarkably, NBTXR3 + XRT + PLT additionally displayed elevated actions in humoral, B cell perform, and antigen processing pathways in comparison with XRT + αPD1(Fig. 4).
Exercise rating of immune pathways within the irradiated tumors. Mice (n = 4) had been handled with numerous mixture therapies as described in Fig. 1 and had been euthanized 11 d put up final fraction of radiation. The RNA from the irradiated tumors was extracted, and the immune-related genes had been measured with a nCounter PanCancer Immune Profiling Panel and a nCounter MAX Evaluation System. The info had been analyzed with the PanCancer Immune Profiling Superior Evaluation Module. P < 0.05 was thought of statistically vital. *P < 0.05, **P < 0.01, ***P < 0.001, NS, not vital
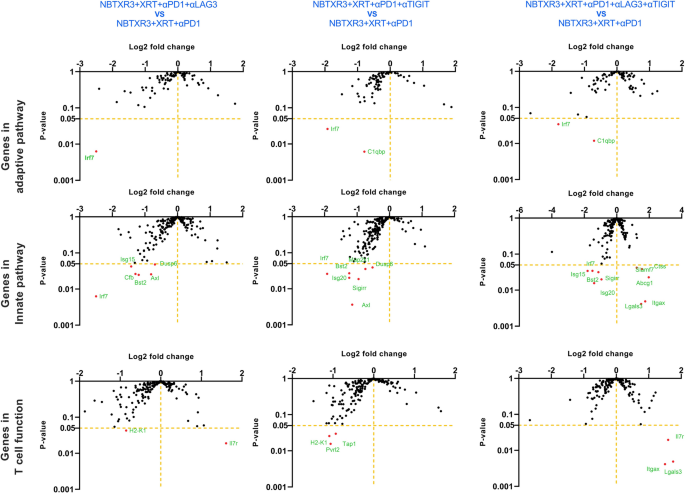
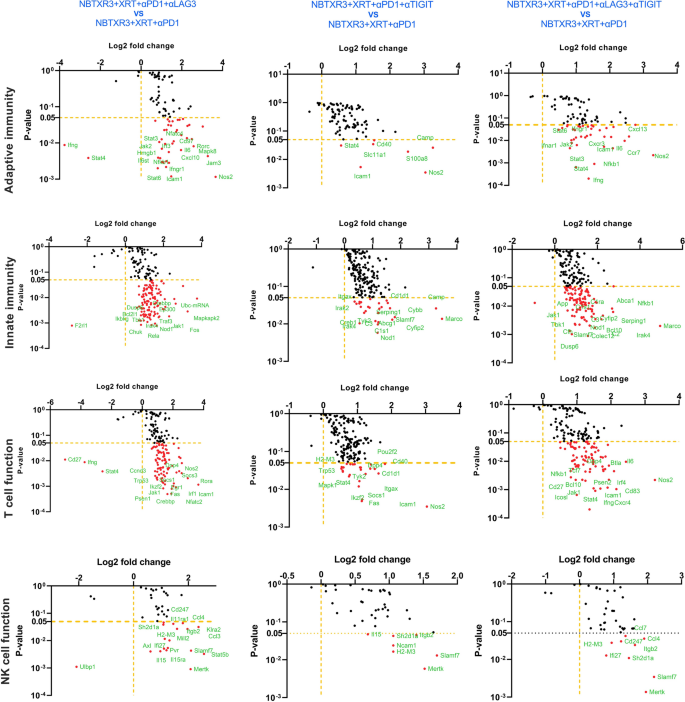
Given the distinctive efficacy we noticed for NBTXR3 + XRT + PLT therapy, we particularly in contrast the immunogenetic profile of main tumors handled with NBTXR3 + XRT + PLT, NBTXR3 + XRT + αPD1 and αLAG3, or NBTXR3 + XRT + αPD1 and αTIGIT as in comparison with these handled with NBTXR3 + XRT + αPD1 alone. On this method, we hoped to see if every iterative addition of blockers induced any further genetic activation. After we thus analyzed the RNA knowledge, we noticed no further upregulation of adaptive immune-related genes with any mixture of blockers above that achieved by NBTXR3 + XRT + αPD1 (Fig. 5). Quite the opposite, there was, in actual fact, a marked downregulation of two immune-related genes: Irf7, which is strongly concerned in antiviral immunity [30], and C1qbp, a element of the complement protein C1q-binding receptor that’s strongly related to the promotion of chemotaxis and metastasis in a number of most cancers sorts [31, 32].
Log2 fold change in expression of genes concerned in adaptive, innate immunity, and T cell perform within the irradiated tumors. Mice (n = 4) had been handled with numerous mixture therapies as described in Fig. 1 and had been euthanized 11 d put up final fraction of radiation. The RNA from the irradiated tumors was extracted, and the immune-related genes had been measured with a nCounter PanCancer Immune Profiling Panel and a nCounter MAX Evaluation System. The info had been analyzed with the PanCancer Immune Profiling Superior Evaluation Module
After we examined innate immune genes, we noticed vital downregulation of much more immune-related genes, all broadly related to activation and irritation (Further file 1: Fig. S8A). These included: Irf7; interferon-stimulated genes Isg15 and 20; complement issue B (Cfb); Axl, a receptor tyrosine kinase that facilitates immune evasion and metastasis in numerous cancers [33, 34]; twin specificity phosphatases Dusp6 and 8; and Map2k1, which is continuously dysregulated in most cancers and is the goal of quite a few experimental inhibitors being developed [35]. Whereas the person genes differed considerably, this downregulation of the inflammatory gene was noticed for all three therapies (Fig. 5).
Nonetheless, in NBXTR3 + PLT alone, we noticed one thing new: a marked upregulation in a number of innate-immune associated genes—very one among which was in concerned with macrophage activation (Further file 1: Fig. S8A). These macrophage-associated genes included: Slamf7, a super-activator of macrophages and a robust promoter of anti-tumor phagocytosis [36]; Abcg1, a macrophage membrane transporter protein that mediates ldl cholesterol efflux, promotes macrophage migration, and restrains irritation and apoptosis [37,38,39]; Lgals3, a cell-cell adhesion mannequin additionally concerned in macrophage activation [40]; cathepsin S (Ctss), a lysosomal protease concerned in peptide catalysis and antigen presentation in macrophages and DCs [41, 42]; and Itgax, a granulocyte integrin which promotes macrophage activation and anti-tumor immunity [43]. The protein product of Itgax, CD11c, additionally serves because the classical marker for antigen-presenting DCs [44]. Taken collectively, the gene expression modifications inside our therapy teams on the main tumor web site level to the downregulation of genetic applications concerned in inflammatory and antiviral-like immunity. Furthermore, when PD1, LAG3, and TIGIT had been inhibited in tandem with NBTXR3-enhanced radiation, there was additionally a sturdy and unambiguous elevation of genes concerned in macrophage activation, enhanced trafficking, tumor phagocytosis, and antigen presentation.
We subsequent examined modifications in immune-related genes within the secondary tumor (unirradiated). In contrast to the first tumor—by which solely genes in pathways related to innate immunity, antigen processing, and B cell perform confirmed upregulation—within the secondary tumor handled with NBTXR2 + XRT + αPD + αTIGIT or αLAG3 or αLAG3 and αTIGIT, we noticed marked, statistically sturdy will increase exercise and gene upregulation in all of the immune pathways, together with adaptive, T cell perform, B cell perform, dendritic cell perform, innate, NK perform, and so forth. (Figs. 6 and 7). This upregulation was probably the most pronounced within the mice handled with NBTXR3 + XRT + PLT (Further file 1: Fig. S8B). Mice handled with NBXTR3 + XRT + αPD1 + αLAG3 universally skilled a broad “smear” of gene expression fold modifications above and under that of untreated controls, presumably indicating extremely dynamic up- and downregulation of a number of genes; nevertheless, most analyzed genes had been upregulated. Upregulation was current—and far sharper and fewer ambiguous—in mice handled with NBXTR3 + XRT + αPD1 + αTIGIT. In each of those mixtures (+αLAG3 and + αTIGIT), some genes had been upregulated above that of the triple remedy. Nonetheless, NBTXR3 + XRT + PLT boasted the “cleanest”, sharpest sign.
Exercise rating of immune pathways within the unirradiated tumors. Mice (n = 4) had been handled with numerous mixture therapies as described in Fig. 1 and had been euthanized 11 d put up final fraction of radiation. The RNA from the unirradiated tumor was extracted, and the immune-related genes had been measured with a nCounter PanCancer Immune Profiling Panel and a nCounter MAX Evaluation System. The info had been analyzed with the PanCancer Immune Profiling Superior Evaluation Module. P < 0.05 was thought of statistically vital. *P < 0.05, **P < 0.01, ***P < 0.001, NS, not vital
Log2 fold change in expression of genes concerned within the adaptive pathway, innate pathway, T cell perform, and NK cell perform within the unirradiated tumors. Mice (n = 4) had been handled with numerous mixture therapies as described in Fig. 1 and had been euthanized 11 d put up final fraction of radiation. The RNA from the unirradiated tumor was extracted, and the immune-related genes had been measured with a nCounter PanCancer Immune Profiling Panel and a nCounter MAX Evaluation System. The info had been analyzed with the PanCancer Immune Profiling Superior Evaluation Module
Given the upregulation of each innate and adaptive pathways – together with pathways concerned in DC perform, antigen processing, T cell perform, and B cell perform, we suspected that what was occurring on the secondary tumor web site was the recruitment of tumor-infiltrating lymphocytes (TILs) that had been primed by macrophages and DCs from the first web site. Within the exploration of this speculation, we carefully examined the person genes that had been statistically considerably (p > 0.05) upregulated within the NBTXR3 + XRT + PLT group and manually grouped them in accordance with perform (Further file 2: Desk S1). We then plotted the combination of the log2 change of every particular person gene from the NBTXR3 + XRT + PLT with the intention to acquire a way of which immune-related pathways had been being altered following therapy (Further file 1: Fig. S8C). Utilizing this evaluation, we discovered that genes concerned in phagocytosis, antigen processing and presentation, cell-cell adhesion, and CD4+ T cell receptor (TCR) signaling had been all elevated within the NBTXR3 + XRT + PLT group in comparison with the NBTXR3 + XRT + αPD1 group. Additionally upregulated had been genes concerned in numerous activating pathways related to the immune response: the JAK-STAT pathway, MAP kinases, IRAKs and TRAFs, and NFκB, in addition to numerous immune-associated tyrosine kinases. The expression of genes encoding quite a few anti-inflammatory cytokines was additionally heightened. Amongst them was IFNγ, produced by activated CD4+ T cells responding to antigen recognition. Briefly, the genetic signature inside the secondary tumor bore the unmistakable mark of sturdy activation of adaptive immunity by antigen presentation.
Additionally current was the genetic signature of a vigorous innate immune response. As beforehand talked about, genes governing phagocytosis—the method whereby innate immune cells, principally macrophages, engulf goal cells—had been extremely upregulated. So too had been genes concerned in reactive oxygen species (ROS) technology, usually upregulated by activated innate immune cells to additional their activation and digesting their prey engulfed by phagocytosis. A number of genes concerned within the complement system, a central mediator of radiotherapy-induced tumor-specific immunity [45], had been additionally upregulated. Among the many most extremely upregulated gene groupings had been these particularly related to macrophage id and performance. The gene for macrophage colony-stimulating issue (Csf1) and its receptor, Csf1r, had been each elevated, as had been the genes for pure resistance-associated macrophage protein 1 (Slc11a1), macrophage receptor with collagenous construction (Marco), and macrophage inflammatory protein 1 β (Ccl4). We, furthermore, noticed an excellent higher upregulation of the macrophage super-activator Slamf7. Altogether, our NanoString knowledge paint an image of sturdy innate and adaptive immune responses on the secondary tumor web site.
NBTXR3 + XRT + PLT therapy produces long-term immunological reminiscence
As demonstrated within the outcomes above, antitumor immune response performs a significant function within the NBTXR3 + XRT + PLT remedy resulted tumor eradication. Essentially the most sturdy immune responses are marked by the event of immunological reminiscence, by which a small remnant of antigen-specific T cells and B cells activated within the preliminary antigen publicity persist, primed, and able to reply quickly ought to the organism ever be challenged by the identical pathogen. The abscopal impact is believed to stimulate such a response, basically changing the first tumor into an in situ vaccine [46].
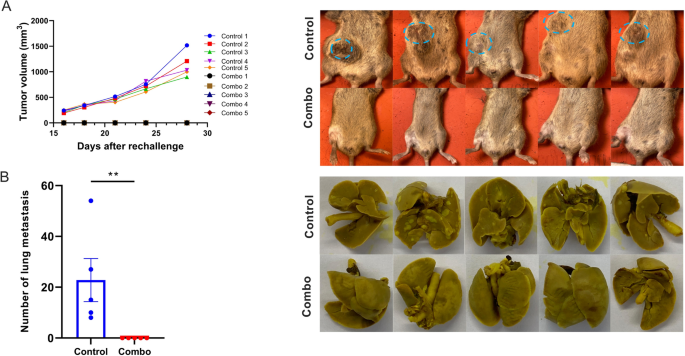
To guage if the cured mice developed an antitumor reminiscence immune response, the 5 survivor mice from the NBTXR3 + XRT + PLT group had been re-injected with 5 × 104 344SQR cells on the correct flank, and their tumor progress was monitored. None of those mice developed tumors (Fig. 8A). In distinction, all mice within the management group did. Twenty-eight days put up tumor re-challenge, when the mice within the management group had reached the experimental endpoint, all mice had been sacrificed, and their lung metastases had been counted. No lung metastasis was noticed within the NBTXR3 + XRT + PLT group (Fig. 8B); nevertheless, all the mice within the management group developed numerous numbers of tumor nodules of their lungs.
NBTXR3 + XRT + PLT (Combo) handled mice reject tumor re-challenge. A Tumor quantity and pictures of the tumors within the mice. B The variety of lung metastases and pictures of the lung tumors. The 5 mice that survived within the NBTXR3 + XRT + PLT group in Fig. 1 had been re-challenged with 5 × 104 344SQR cells on the correct flank at the very least 60 d after the final fraction of 12 Gy. 5 mice with 5 × 104 344SQR cells inoculated on the correct flank served because the management. All of the mice had been euthanized 28 d after tumor inoculation. Lungs had been harvested from the mice, and the variety of lung metastases was counted. P < 0.05 was thought of statistically vital. **P < 0.01, NS, not vital
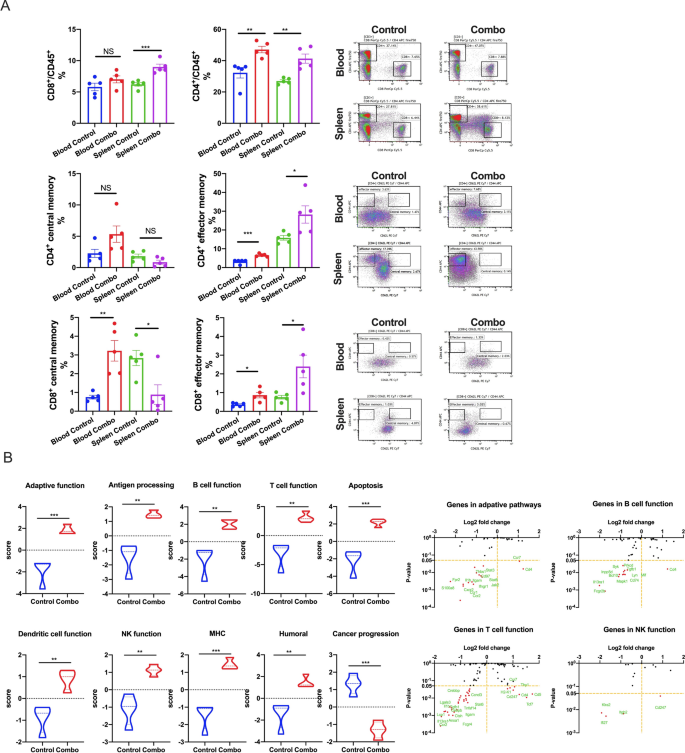
We subsequent regarded immediately on the ranges of complete, central, and effector reminiscence T cells (Fig. 9A). We collected blood and spleens from each experimental teams, from which we remoted CD4+ and CD8+ reminiscence T cells. The variations in reminiscence composition and distribution had been broadly related between each T cell subsets. For each CD4+ and CD8+ cells, complete (CD45+) and effector reminiscence T cells (TEM cells; CD44+CD62L–) had been elevated inside the blood and spleens of NBTXR3 + XRT + PLT-treated mice (although this elevation was not statistically vital for blood complete reminiscence CD8+ T cells). Central reminiscence T cells (TCM cells; CD44+CD62L+) of each compartments had been enriched within the blood – considerably so within the case of CD8+ T cells; curiously, nevertheless, TCM cells of each compartments had been much less considerable inside the spleens of handled mice than controls (once more, this was statistically vital for CD8+ T cells, not so for CD4+). Given the context of this commentary (i.e., throughout an ongoing immune problem), we suspect that these numbers paint the image of a poised reminiscence response that has been sprung, triggering a mass re-activation of TCM cells of each subsets, their differentiation into TEM cells [47], and their exodus from the spleen into the bloodstream.
NBTXR3 + XRT + PLT (Combo) induces immunological reminiscence and upregulates immune actions within the blood. A Percentages of CD4+/CD45+ and CD8+/CD45+ T cells and reminiscence CD4+ and CD8+ T cells within the blood and spleen. B Nanostring exercise rating of immune pathways and log2 fold change in expression of genes concerned within the adaptive pathway, NK cell perform, B cell perform, and T cell perform with the management because the baseline. The 5 mice that survived within the NBTXR3 + XRT + PLT group in Fig. 1 had been re-challenged with 5 × 104 344SQR cells on the correct flank at the very least 60 d after the final fraction of 12 Gy. 5 mice with 5 × 104 344SQR cells inoculated on the correct flank served because the management. All of the mice had been euthanized 28 d after tumor inoculation. Blood was harvested earlier than euthanizing the mice, pink blood cells had been lysed, and RNA was extracted for Nanostring evaluation. The populations of reminiscence CD4+ and CD8+ T cells had been analyzed by stream cytometry. P < 0.05 was thought of statistically vital. *P < 0.05, **P < 0.01, ***P < 0.001, NS, not vital
Lastly, we examined peripheral blood monocytes (PBMCs) remoted for gene upregulation utilizing the identical Nanostring module beforehand described. PBMCs from mice handled with NBTXR3 + XRT + PLT exhibited vital upregulation in each immune-related genetic program examined, and a big downregulation of genes related to most cancers development (Fig. 9B). This, in coordination with the dearth of any tumor growth and the hardy reminiscence T cell response, signifies that mice handled with NBTXR3 + XRT + PLT weren’t solely capable of survive and clear the preliminary tumor problem, however had been capable of develop immunological reminiscence that inoculated them towards the identical tumor kind.