Synthesis and characterization of Fe-CDs
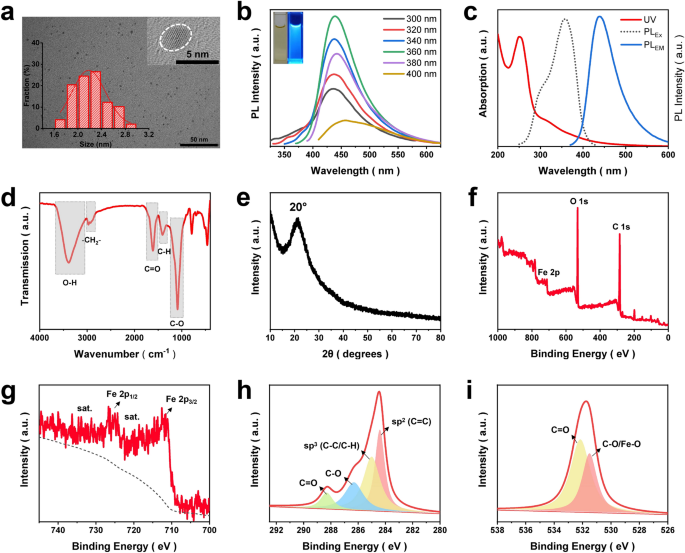
Within the current work, Fe-doped carbon dots (Fe-CDs) had been derived from a Meals and Drug Administration (FDA)-approved inexperienced iron complement, i.e., ferrous gluconate, through an environmentally pleasant one-step hydrothermal response. Our earlier stories revealed that this type of gluconic acid complex-based steel salt was appropriate for the preparation of steel ions-doped CDs (MCDs) [34, 35]. Lately, MCDs had proven rising bioapplication prospects within the fields of analysis and remedy, because of the synergistic options of the nano-size impact, biocompatibility of CDs and particular performance of steel ions [36]. Iron metabolism within the tumor-related cells was carefully associated to the prevalence and growth of most cancers, which impressed loads of works on Fe-based anti-tumor nanomaterials, bringing most cancers remedy into an iron age [37,38,39]. On this regard, it was speculated that the as-synthesized Fe-CDs may need potential as a novel iron agent for tumor therapy. First, the morphology and construction of Fe-CDs had been investigated, as proven in Fig. 1a, the transmission electron microscopy (TEM) picture confirmed that Fe-CDs appeared as uniform nanoparticle with a median diameter of two.2 nm, such a small nanosize would facilitate the speedy entry of Fe-CDs into cells. The high-resolution TEM picture revealed lattice fringes with 0.21 nm spacing, which was equivalent to the (100) crystal aircraft of graphite, indicating the graphitized carbon core inside Fe-CDs [40] The photoluminescence (PL) spectrum of Fe-CDs exhibited an typical excitation wavelength-dependent blue fluorescence property (Fig. 1b) [41], and the utmost emission wavelength was 440 nm when excited below 358 nm mild (Fig. 1c). There have been two peaks noticed within the UV–Vis absorption spectra of Fe-CDs that positioned at 252 and 316 nm, which could possibly be attributed to the π → π* transition of C = C bond and n → π* transition of C = O bond, respectively [42]. Fourier remodel infrared spectroscopy (FTIR) was adopted to check the practical teams of Fe-CDs, the stretching vibrations of O–H at 3386 cm−1, C = O at 1610 cm−1 and C-O at 1089 cm−1 (Fig. 1d), demonstrating the plentiful oxygen-containing teams on the floor of Fe-CDs that endow it with good water dispersibility.
Characterization of Fe-CDs. a TEM photos of Fe-CDs, insets present the scale distribution histogram and the HR-TEM picture. b PL spectra of Fe-CDs resolution below completely different excitation wavelengths. c UV–Vis absorption spectra, the utmost excitation and emission PL spectra of Fe-CDs. d FT-IR spectrum of Fe-CDs. e XRD sample of Fe-CDs. f XPS full spectra of Fe-CDs. g XPS deconvoluted Fe 2p spectra. h XPS deconvoluted C 1 s spectra, i XPS deconvoluted O 1 s spectra
Then, the prevailing states of Fe inside Fe-CDs was additional decided. X-ray diffraction evaluation of Fe-CDs displayed a broad peak at round 20°, equivalent to amorphous carbon (Fig. 1e) [43]. There have been no attribute peaks of iron oxide or elemental iron, which is likely to be brought on by the low iron content material, or presumably on account of the truth that Fe dopants in Fe-CDs had been ionic type. This was presumably as a result of the hydrothermal circumstances had been comparatively gentle and the insoluble oxide from the as-prepared merchandise had been additionally subjected to post-treatment by filtration. The existence of Fe in Fe-CDs was confirmed by power dispersive spectrometer (EDS), which decided the factor weight share was 72.1%, 18.2%, and 9.7% for C, O, and Fe, respectively (Further file 1: Fig. S1). Apart from, the Fe ion content material was additionally precisely analyzed as 6.46 wt% by inductive coupled plasma emission spectrometer (ICP) (Further file 1: Fig. S2), just like the EDS end result. With a purpose to additional perceive the basic composition of Fe-CDs and doping types of Fe, X-ray photoelectron spectroscopy was performed, which confirmed the existance of C, O, and Fe in Fe-CDs with the atomic share (At.%) of 64.9%, 28.9%, and 6.2%, respectively (Fig. 1f). The deconvoluted Fe 2p spectra of Fe-CDs possessed the indicators of Fe 2p3/2 and Fe 2p1/2 digital configurations on the binding power of 711.6 and 724.9 eV, respectively, together with the adjoining satellite tv for pc peaks (Fig. 1g). These had been related to the presence of ionic Fe3+ in keeping with earlier stories [44], which additional confirmed the XRD evaluation. The C 1 s spectra was divided into 4 kinds of carbon, i.e., sp2 C (C = C), sp3 C (C–C), C-O, and C = O at 284.4, 285.1, 286.3, and 288.4 eV, respectively (Fig. 1h), which was in step with the FTIR outcomes. The deconvoluted peaks within the O 1 s spectra at 532.2 and 531.5 eV could possibly be ascribed to C = O and C-O/Fe–O bonds, respectively (Fig. 1i). Furthermore, the chromatic response of Fe3+ and salicylic acid (SA) was launched to find out the iron-doped state in Fe-CDs (Further file 1: Fig. S3). It could possibly be clearly seen that with the rise of the focus of Fe-CDs added in SA resolution, the purple purple complicated shade deepened. Nevertheless, there appeared no shade change after the blending of divalent iron salt FeSO4 and SA, indicating the Fe3+ doping standing of Fe-CDs. In abstract, a kind of Fe ions-doped fluorescent CDs was efficiently synthesized, during which Fe ions could possibly be sure to oxygen-containing practical teams on the floor of CDs by means of electrostatic interactions or ionic bonds. It will be of nice curiosity to research the function of this novel nano-iron agent within the regulation of TME and the applying in anti-tumor.
In vivo analysis of anti-tumor results of Fe-CDs
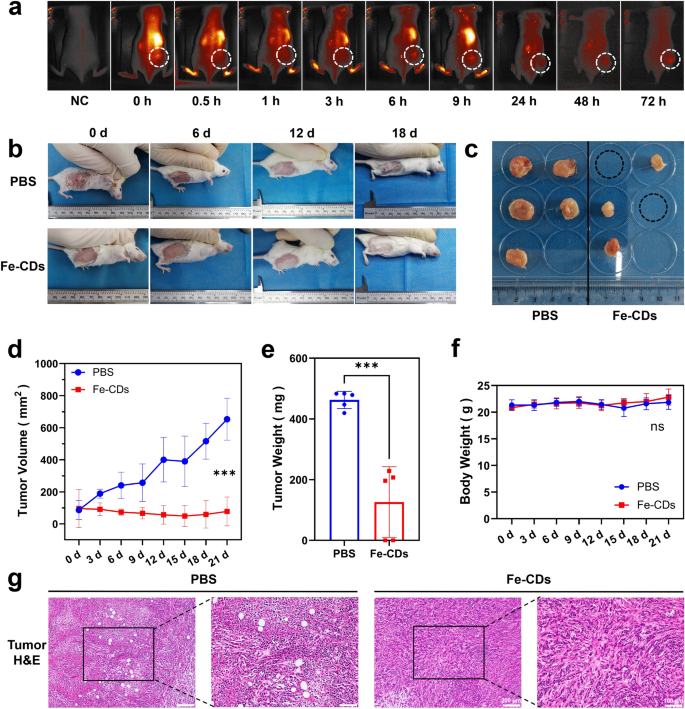
An orthotopic tumor mannequin was constructed by subcutaneous injection of 4T1 cells (mouse breast most cancers cells) at the back of Balb/C mice, which was a basic tumor mannequin appropriate for preliminary analysis of the Fe-CDs anti-tumor impact. Initially, the in vivo metabolic kinetics of Fe-CDs was studied by intravenous injection of Fe-CDs chemically coupled with Cyanine 5.5 (Cy5.5), which may obtain near-infrared intravital imaging with low interference of tissue autofluorescence. As proven in Fig. 2a, the fluorescence sign was primarily concentrated within the liver space of mouse throughout the first 3 h, in step with the earlier stories that CDs being metabolized by liver and kidney [45]. Then, Fe-CDs progressively collected to the tumor website from 6 h after injection till 72 h (white dashed space), which could possibly be because of the enhanced permeability and retention impact (EPR) impact of nano-sized Fe-CDs, or the elevated permeability of tumor blood vessels resulting in the native uptake of Fe-CDs [46].
The in vivo analysis of anti-tumor exercise of Fe-CDs. a Distribution of Fe-CDs in tumor-bearing mice utilizing an in vivo imaging system. b Pictures of tumor-bearing mice at completely different therapy instances of PBS and Fe-CDs. c Pictures of tumors collected from completely different teams of mice after 21 d therapy of PBS (left) and Fe-CDs (proper). d Imply tumor quantity versus time curve of PBS and Fe-CDs therapy teams. e Imply weight of tumors of PBS and Fe-CDs therapy teams at 21 d. f Imply physique weight of regular mice after intravenous administration of PBS and Fe-CDs for various days. g Histological evaluation of cancerous tissues by H&E staining after remedies with PBS and Fe-CDs. (significance evaluation was carried out utilizing one-way ANOVA methodology. ns: not vital, ***p < 0.001, n = 6)
The circulating administration of Fe-CDs (2 mg/20 g physique weight per day, injected as soon as each 3 d) by means of tail vein was began when the subcutaneous tumor reached about 100 mm3, and the management group was an equal quantity of PBS buffer resolution injected intravenously (Fig. 2b). As we anticipated, the diameter of the resected tumor had grown to 12.56 ± 1.97 mm within the management group, whereas the tumor measurement of Fe-CDs group decreased to six.74 ± 1.48 mm. Surprisingly, the tumors had fully disappeared in two teams of tumor-bearing mice (Fig. 2c), demonstrating the superior skill of Fe-CDs to withstand tumors. The imply tumor quantity progressively decreased and finally shrank to about half of the preliminary worth when Fe-CDs had been administered each 3 days (Fig. 2d). Nevertheless, the tumor quantity within the PBS-injected group elevated by greater than 5 instances. Additionally, the common tumor weight of the PBS group was about 3.7 instances that of the Fe-CDs therapy group, indicating that Fe-CDs considerably inhibited the in-vivo proliferation of strong tumors (Fig. 2e).
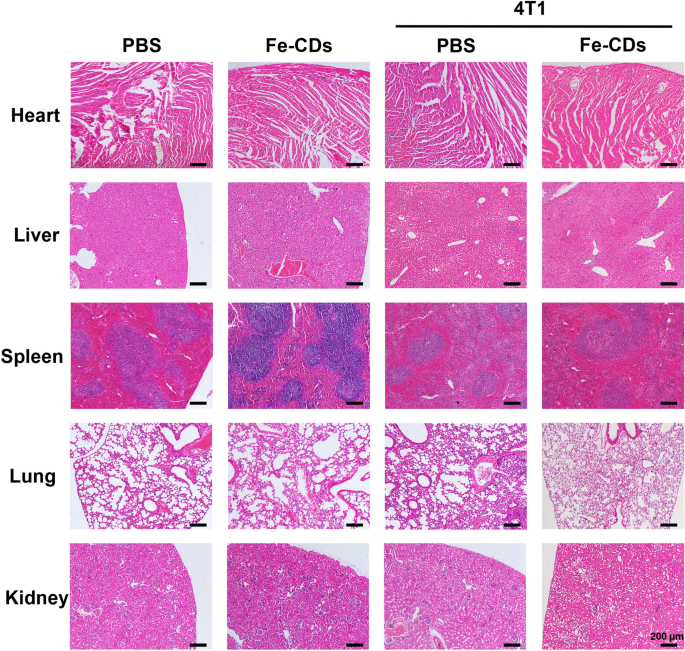
Furthermore, systemic circulatory dosing of Fe-CDs had no vital influences on the mice weight, which demonstrated the extraordinarily low toxicity of Fe-CDs (Fig. 2f). The hematoxylin–eosin staining (H&E) of tumor tissue illustrated that there have been apparent necrotic areas and considerably remission of immune setting inside the tumor space in Fe-CDs group in contrast with the PBS group, confirming that Fe-CDs may selectively induce tumor necrosis and ablation (Fig. 2g). It was value mentioning that such an anti-tumor impact could possibly be achieved by intravenous administration of Fe-CDs, with out integrating different chemotherapy or phototherapy moieties, and concentrating on molecules. With a purpose to additional confirm the biocompatibility of Fe-CDs to regular tissues, the very important organs (coronary heart, liver, spleen, lung, kidney) of mice had been dissected out for H&E staining, and the sections displayed no apparent modifications of cell states and inflammatory infiltration in each the management group and Fe-CDs group, which additionally proved the superb biocompatibility of Fe-CDs (Fig. 3). Due to this fact, a kind of biocompatible Fe-CDs with distinctive anti-tumor exercise was merely synthesized, which confirmed nice potential as a target-free, low-toxicity, and efficient anti-tumor nanodrug. It was essential to discover and make clear the mechanism for the environment friendly tumor therapy impact of Fe-CDs, in order to offer steering and reference for the preparation of Fe-based anti-tumor nanomaterials.
Cytotoxicity and talent of Fe-CDs to induce tumor cell apoptosis
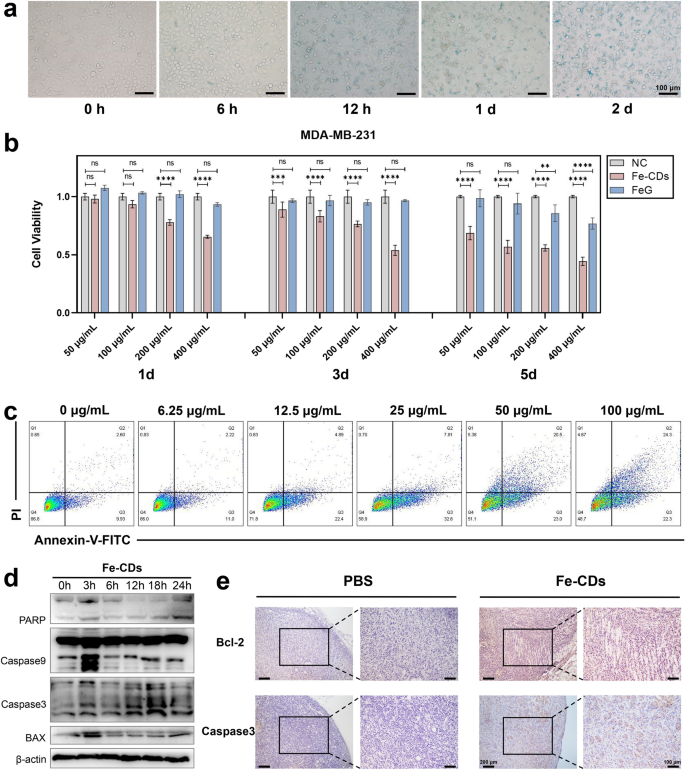
Earlier than conducting in vitro anti-tumor associated experiments, the mobile localization and cytotoxicity wanted to be assessed. Prussian blue staining was used to find Fe-CDs in human triple-negative breast most cancers cells (MDA-MB-231) by labeling ferric irons (Fig. 4a). After 6 h of co-culture with Fe-CDs, there have been seen blue markers round partial cells, confirming preliminary mobile uptake of Fe-CDs. As well as, some cells confirmed morphological modifications, nuclear pyknosis, and destruction of cell membrane after 12 h. With the extension of incubation time until 48 h, the content material of Fe-positive labeled cells and necrotic cells progressively elevated, which steered that Fe-CDs could possibly be enriched in MDA-MB-231 cells and induce cell dying. Furthermore, TEM photos of tumor cell sections that co-cultured with Fe-CDs additionally clearly confirmed the presence of Fe-CDs within the state of aggregated particles inside the cytoplasm in 2 h (Further file 1: Fig. S4), indicating that they may enter the tumor cells rapidly.
The impact of Fe-CDs on tumor cell viability and apoptosis. a Prussian blue staining of Fe in tumor cells incubated with Fe-CDs for various time. b Cell viability of tumor cells incubated with Fe-CDs and FeG by MTT assay. c Cell apoptosis of tumor cells incubated with completely different focus of Fe-CDs by stream cytometry. d Western blot of expression ranges of apoptosis-related proteins in tumor cells handled with Fe-CDs at completely different incubation time. e Immunohistochemical staining of apoptosis marker in cancerous tissues handled with PBS (left) and Fe-CDs (proper). (ns: not vital, **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 6)
Then, the cytotoxicity experiment of Fe-CDs was carried out by MTT assay, and the uncooked materials ferrous gluconate was used as a management to outline the precise function of getting ready Fe-CDs. It could possibly be seen that Fe-CDs considerably led to MDA-MB-231 cell dying with a time- and concentration-dependent method (Fig. 4b), when the tradition situation was 400 μg/mL of Fe-CDs for 3 d and 100 μg/mL for five d, the cell viability reached (53.8 ± 0.031)% and (55.9 ± 0.019)%, respectively, in different phrases, practically half of the most cancers cells died below Fe-CDs therapy. In distinction, the uncooked materials compound, i.e., ferrous gluconate (FeG), had nearly no cytotoxicity to tumor cells, just like the management group. It was speculated that because of the improved water solubility and cell entry mode of iron medicine after nanometerization, which made it simpler to be taken up by cells, and the publicity of iron on the floor of carbon dots was additionally conducive to its impact. It was value mentioning that Fe-CDs may selectively kill tumor cells with out exhibiting apparent toxicity to regular cells, reminiscent of human dental pulp stem cells (hDPSCs) and human umbilical vein cell fusion cells (EA.hy926), with cell viability above 80% in any respect concentrations and time gradients (Further file 1: Fig. S5–S6), indicating the the potential of Fe-CDs as biocompatible antitumor medicine.
With a purpose to verify how Fe-CDs brought on tumor cell dying, the consequences of various inhibitors on cell exercise had been investigated. The cell ferroptosis inhibitor (Fer) and necrosis inhibitor (Nec) confirmed restraining results on the lower of cell viability brought on by Fe-CDs, particularly cell apoptosis inhibitor (ZVAD) considerably reversed the Fe-CDs induced cell dying (Further file 1: Fig. S7), indicating that Fe-CDs kill tumor cells primarily by means of apoptosis pathway. Usually, ferroptosis was primarily brought on by lipid peroxidation catalyzed by divalent Fe ions [47]. Though the as-prepared Fe-CDs was a kind of Fe-doped nanomaterials, the Fe inside Fe-CDs existed primarily in Fe3+ type, so the tumor cell dying brought on by Fe-CDs was not primarily by means of ferroptosis method. As well as, the doping quantity of Fe in Fe-CDs was comparatively low, it was believed that the nano-structures and floor teams of CDs additionally performed an essential synergistic function within the improved anti-tumor perform and biocompatibility of Fe-CDs. Additional, Annexin-V and PI double staining was launched to detect apoptosis by stream cytometer, which was proven in Fig. 4c. When the focus of Fe-CDs was low (< 25 μg/mL), early apoptosis of MDA-MB-231 cells primarily occurred (Q3) and the apoptosis fee elevated with the rise of Fe-CDs focus. Then late apoptosis began to rise (Q2) and have become dominant because the focus of Fe-CDs additional elevated to 100 μg/mL, representing that the development of apoptosis was exacerbated by excessive Fe-CDs focus. It was additional verified by Western Blot that Fe-CDs induce tumor cell apoptosis fairly than different dying pathways. The addition of Fe-CDs activated the expression of apoptosis-related protein together with PARP, Caspase 3, Caspase 9, and BAX with a time-dependent attribute (Fig. 4d, Further file 1: Fig. S8). Furthermore, immunohistochemical staining of apoptosis-related markers was carried out in tumor tissue sections, specifically B-cell CLL/lymphoma 2 (Bcl-2) and Caspase 3, to additional decide the power of Fe-CDs in inducing tumor apoptosis. The up-regulation of apoptosis degree in Fe-CDs group could possibly be clearly noticed as in comparison with the damaging management (NC) group (Fig. 4e). It’s value noting that G-CDs with out Fe doping didn’t have tumor cytotoxicity and did not induce apoptosis of tumor cells (Further file 1: Fig. S9–S11), indicating that the power of Fe-CDs to trigger tumor cell dying is inseparable from Fe dopants. The above outcomes demonstrated that Fe-CDs possessed the perform of inducing tumor cell apoptosis, which contributed to its wonderful anti-tumor impact in vivo.
Means of Fe-CDs to activate the immunity of antitumoral macrophages
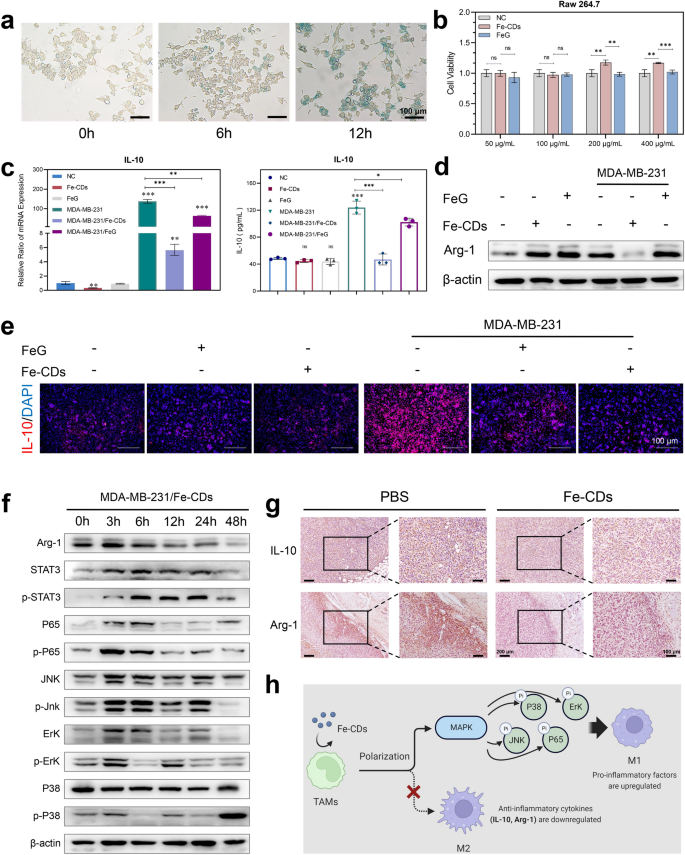
Immunotherapy had grow to be essentially the most promising anti-cancer therapies within the clinic as a result of it utilized the mobilization of autoimmune techniques to realize tumor eradication with extraordinarily low unwanted side effects and recurrence charges. In tumor microenvrionment (TME), Fe was preferentially taken up by the encircling tumor-associated macrophages (TAMs), forming iron-carrying macrophages [48, 49]. Earlier stories had discovered that an FDA-approved iron agent, i.e., ferumoxytol (nano-iron oxide), may inhibit tumor cell proliferation by modulating macrophages features [39, 50]. Relating to this, the consequences of Fe-CDs on TAMs immunity had been additional explored. Equally, the localization of Fe-CDs in mouse mononuclear macrophage leukemia cells (RAW 264.7) was additionally achieved by Prussian blue staining, exhibiting that Fe-CDs started to enter cells inside 1 day, and the diploma of entry additional elevated at 3 days (Fig. 5a), which confirmed that Fe-CDs could possibly be enriched in macrophages. Unexpectedly, each Fe-CDs and FeG confirmed no vital cytotoxicity to macrophages in a sure focus vary (Fig. 5b), that’s, Fe-CDs may selectively kill tumor cells with out unwanted side effects on other forms of cells, and this efficiency was extraordinarily essential for the sensible software of anti-tumor nanomedicines.
The power of Fe-CDs to modulate macrophage polarization. a Prussian blue staining of Fe in macrophages incubated with Fe-CDs for various time. b Cell viability of macrophages incubated with Fe-CDs and FeG by MTT assay. c qPCR assay (left) and ELISA assay (proper) of IL-10 expression degree in macrophages with (proper) and with out (left) tumor cell medium, within the presence of Fe-CDs or FeG. d Western blot of Arg-1 expression ranges in macrophages incubated with (proper) or with out (left) tumor cell medium, within the presence (+) or absence (−) of Fe-CDs and FeG. e Immunofluorescence photos of IL-10 in macrophages with or with out tumor cell medium, within the presence or absence of Fe-CDs and FeG. f Western blot of expression ranges of M2-type macrophage-related proteins in macrophages handled with tumor cell medium within the presence of Fe-CDs at completely different incubation time. g Immunohistochemical staining of M2-type macrophage marker in cancerous tissues handled with PBS (left) and Fe-CDs (proper). h Schematic illustration of the power of Fe-CDs in modulating macrophage polarization (Created with BioRender.com). (ns: not vital, *P < 0.01, **P < 0.001, ***P < 0.0001, n = 6)
Then, quantitative real-time polymerase chain response (qPCR) and enzyme linked immunosorbent assay (ELISA) had been carried out to research the expression degree of Interleukin-10 (IL-10) in macrophages below the presence of Fe-CDs. The cytokine IL-10 was discovered to be abundantly secreted by activated macrophages in TME, making the phenotype of TAMs are usually anti-inflammatory M2, which may inhibit the immune response and particular tumor killing [51, 52]. Right here, it could possibly be seen that Fe-CDs had no vital impact on IL-10 era in macrophages below regular circumstances (Fig. 5c). Nevertheless, when macrophages cultured in tumor cells tradition medium had been used to imitate TAMs, the mRNA transcription and protein expression of IL-10 had been clearly down-regulated by the addition of Fe-CDs. In different phrases, the differentiation of pro-inflammatory M1 kind TAMs was promoted by means of inhibiting IL-10 secretion by Fe-CDs, which may awaken the immune perform of antitumoral macrophages to selectively kill tumor cells. Noteworthy, the primitive drug FeG confirmed a comparatively weaker exercise than Fe-CDs because of the poor solubility and uptake charges. Additionally, Fe-free G-CDs didn’t have the aptitude to reverse the expression of IL-10 in TAMs (Further file: Determine S12), indicating the significance of each Fe doping and CDs nanocarrier for Fe-CDs to activate macrophages immunity. The outcomes of immunofluorescence staining additional proved the conclusion that the tumor cell tradition considerably stimulate macrophages to secrete IL-10, which could possibly be successfully restrained by Fe-CDs (Fig. 5d). Furthermore, one other two typical soluble mediator secreted by M2 macrophages, Arginase-1 (Arg-1) and phosphorylation of P38 (p-P38), may function the precise markers for M2 polarization [52, 53]. It could possibly be clearly seen that the presence of Fe-CDs considerably decreased the expression of Arg-1 and p-P38 in macrophages cultured in tumor cell medium, whereas FeG had nearly no impact on Arg-1 and p-P38 ranges (Fig. 5e, Further file 1: Fig. S13–S14). As well as, in contrast with untreated TAMs (Further file 1: Fig. S15–S16), Fe-CDs additionally markedly inhibited the phosphorylation ranges of sign transducer and activator of transcription 3 (STAT3), c-Jun N-terminal kinase (p-JNK), extracellular regulated protein kinases (ERK), and P65 with a time-dependent tendency (Fig. 5f, Further file 1: Fig. S17), which each belonged to the Mitogen-activated protein kinases (MAPK) signaling pathway. Based on the earlier stories, the activation of MAPK was important for the antitumoral macrophages [54]. Furthermore, the in vivo histochemical staining outcomes of IL-10 and Arg-1 in tumor tissues additional demonstrated that Fe-CDs stimulated antitumoral macrophages by inhibiting the expression of IL-10/Arg-1 (Fig. 5g). Primarily based on the above outcomes, it was concluded that the as-prepared Fe-CDs may modulate tumor immunity by the macrophage IL-10/Arg-1 routes, and promoted the transformation of macrophages to the tumor-specific killer M1 phenotype by means of the MAPK pathway (Fig. 5h), particularly the regulating skill was stronger than that of FeG because of the improved internalization effectivity after nanoization.
Means of Fe-CDs to inhibit epithelial-mesenchymal transition (EMT) course of
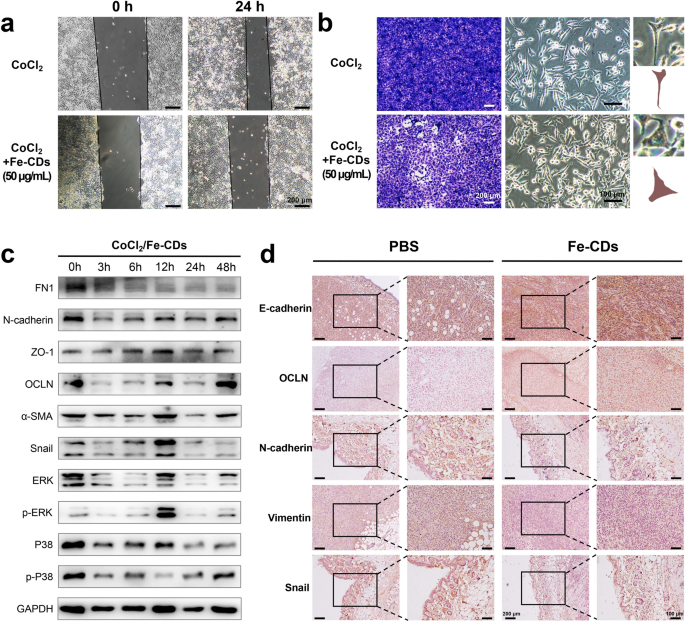
The metastasis of tumor cells, which led to the recurrence and unfold of malignant tumors, had been thought-about as crucial issue within the incurability of most cancers [55]. Lately, it was discovered that the programmed activation of epithelial-mesenchymal transition (EMT) may make tumor cells lose the tight junction and adhesion between cells, after which switched to the morphology of mesenchymal cells with sturdy migratory skill [56]. Surprisingly, the as-synthesized Fe-CDs possessed the distinctive skill to inhibit tumor cell migration and EMT. First, the scratch assay photos of MDA-MB-231 cells cultivated with Fe-CDs exhibited a big discount of proliferation fee in comparison with the management group that incubated in phosphate buffered saline (PBS) (Further file 1: Fig. S18a). The migration fee of the management group in scratch assay was 57%, whereas the migration fee of cells handled with Fe-CDs was decreased to nearly 6% (Further file 1: Fig. S19a). Noteworthy, G-CDs with out Fe doping didn’t have the power to inhibit tumor cell migration (Further file 1: Fig. S20), that’s, the perform of Fe-CDs to forestall EMT was associated to the doped Fe, which is likely to be because of the cost repulsion of Fe ions or the affect of EMT-related signaling pathways. Then, the morphology of tumor cells noticed below microscope modified from cobblestone-like to spindle-like form, indicating the lack of cell polarity and elevated migratory capability (Further file 1: Fig. S18b). Nevertheless, the existence of Fe-CDs (50 μg/mL) may partially reverse the cytoskeletal modifications to a sure extent, representing its potential in suppressing EMT of tumor cells. As well as, cobalt chloride (CoCl2) was launched to additional discover the perform of Fe-CDs in suppressing EMT, as an applicable quantity of CoCl2 had been reported to induce mesenchymal cell transformation [57]. Much like the above experiments, it could possibly be seen that though CoCl2 accelerated the cell migration course of, the addition of Fe-CDs may nonetheless successfully stop tumor cells from infiltrating into the scratch (Fig. 6a). The migration fee of cells handled with CoCl2 was 50%, whereas the migration fee of tumor cells co-treated with CoCl2 and Fe-CDs was decreased to eight% (Further file 1: Fig. S19b). Additionally, the exacerbated strategy of cell morphological transformation was alleviated by Fe-CDs, that’s, within the presence of Fe-CDs, the tumor cells could possibly be transformed from aggressive spindle form to conservative spherical form (Fig. 6b). These outcomes had been additional confirmed by the transwell migration experiments proven on the left aspect of Fig. 6b, which clearly depicted that the variety of chemotactic tumor cells in each the management and CoCl2 teams was considerably increased when Fe-CDs was absent.
The impact of Fe-CDs on the EMT of tumor cells. a Scratch check of the impact of CoCl2 and Fe-CDs on the migration skill of tumor cells. b Transwell assay (left) and cell morphology photos (proper) of the impact of CoCl2 and Fe-CDs on the migration skill of tumor cells. c Western blot of expression ranges of EMT-related proteins in tumor cells handled with CoCl2 and Fe-CDs at completely different incubation time. d Immunohistochemical staining of EMT marker in cancerous tissues handled with PBS (left) and Fe-CDs (proper)
Moreover, a number of EMT-related markers had been carried out by WB evaluation, together with Fibronectin (FN1), N-cadherin, Vemintin, α-Easy muscle actin (α-SMA), Snail, which had been reported to induce EMT, and in addition Zonula occludens-1 (ZO-1), Occludin (OCLN), which may inhibit EMT (Further file 1: Fig. S21) [58]. It could possibly be seen that Fe-CDs therapy considerably decreased the expression of EMT positive-associated proteins and confirmed a rise within the expression degree of EMT inhibitory proteins with a concentration-dependent method (Further file 1: Fig. S22). The apparent up-regulation of p-P38 and p-ERK with the addition of Fe-CDs additionally steered that EMT course of is likely to be inhibited by means of associated pathways. Equally, when CoCl2 was launched, the above proteins had been additionally subjected to WB check, which confirmed that they may speed up the EMT strategy of tumor cells over time (Further file 1: Fig. S23), whereas Fe-CDs successfully counteracted the progress of EMT (Fig. 6c, Further file 1: Fig. S24–S25). Moreover, the perform of Fe-CDs to inhibit EMT was additionally verified in vivo by staining with the antibodies of E-cadherin, OCLN, N-cadherin, Vimentin and Snail, just like the WB assay in cells (Fig. 6d). The above outcomes demonstrated that Fe-CDs may need the potential to suppress most cancers migration by blocking the EMT strategy of tumor cells. Because the as-synthesized Fe-CDs had been proved to be outfitted with three-step most cancers remedy features, i.e., inducing tumor cell apoptosis, modulating macrophage immunity, and inhibiting EMT, resulting in the superior anti-tumor impact of Fe-CDs in vivo with out integrating different moieties and exterior stimuli, it was believed that Fe-CDs may function a promising various to tumor immunotherapy brokers and anti-tumor nanoplatform.