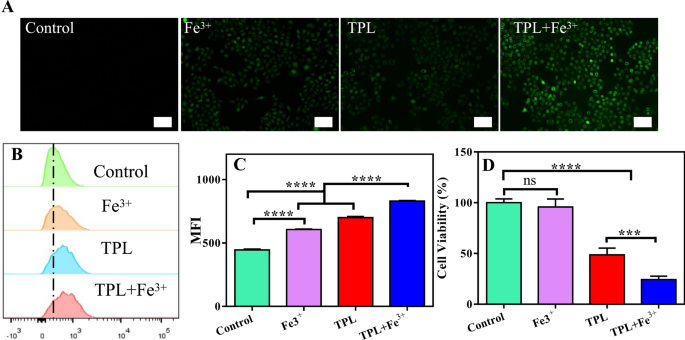
Results of the mixture of Fe3+ and TPL on ROS and cytotoxicity
It has been reported that TPL can inhibit the expression of Nrf2, thus inhibiting Nrf2-mediated GSH synthesis and growing intracellular ROS ranges [28]. As well as, the rise in intracellular iron can improve ROS manufacturing by means of the Fenton response [23]. Due to this fact, we thought-about whether or not the mixture of Fe3+ and TPL can synergistically amplify ROS technology. First, intracellular ROS ranges have been measured utilizing DCFH-DA probe. DCFH-DA itself isn’t fluorescent, however it may be quickly oxidized within the presence of ROS to supply fluorescent compound 2′,7′-dichlorofluorescein (DCF), and thus the degrees of ROS will be monitored dynamically by measuring the depth of fluorescence sign. As predicted, there was weak fluorescence within the untreated group, indicating basal ranges of intracellular ROS. The fluorescence depth was enhanced within the Fe3+ group in comparison with the management group, indicating that Fe could improve the manufacturing of intracellular ROS. As well as, the fluorescence depth of the TPL group was additionally enhanced in contrast with the management group, indicating that TPL might additionally induce ROS manufacturing. Surprisingly, the fluorescence depth of the TPL plus Fe3+ teams was additional enhanced, suggesting that Fe3+ might cooperate with TPL to extend ROS manufacturing after getting into cells (Fig. 1A). For quantitative evaluation, we measured the intracellular fluorescence depth by circulate cytometry (Fig. 1B, C), and these knowledge have been in keeping with the outcomes of fluorescence micrographs. This exhibits that the mixture of Fe and TPL has a major capacity to synergistically amplify ROS manufacturing. Then, we initially evaluated the toxicity of Fe3+ together with TPL to B16F10 cells utilizing CCK8 assays (Fig. 1D). We discovered that Fe3+ alone confirmed virtually no toxicity to B16F10 cells, and though Fe3+ was beforehand proven to advertise intracellular ROS ranges, it was not adequate to inhibit cell development. Notably, Fe3+ together with TPL exhibited stronger cytotoxicity than free TPL, suggesting that TPL together with Fe3+ can synergistically amplify ROS and thus exert anti-tumor impact.
A Fluorescence microscopy pictures displaying ROS ranges in B16F10 cells handled with completely different brokers. Scale bar: 50 μm. B Movement cytometry outcomes of the ROS ranges in B16F10 cells handled with completely different formulations. C The typical fluorescence depth of various formulations incubated with B16F10 cells. MFI: Imply fluorescence depth. Knowledge are expressed because the imply ± SD (n = 3). ns, not vital, ****P < 0.0001. D Viability of B16F10 cells incubated with completely different formulations for 48 h
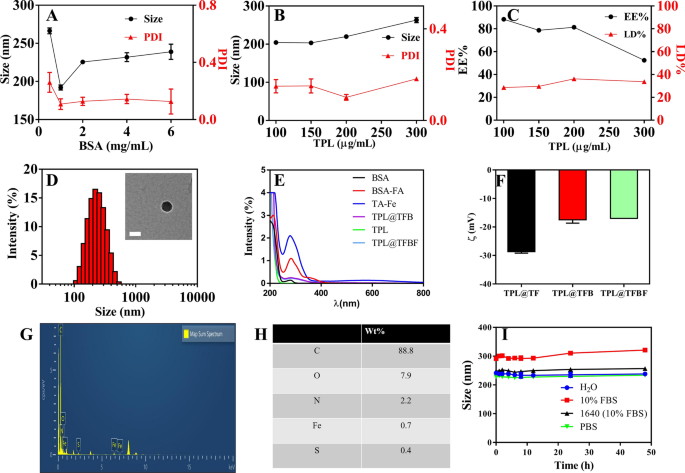
Preparation and characterization of TPL@TFBF
TPL is a hydrophobic drug with poor solubility in aqueous answer. Moreover, the power of free Fe3+ to enter the cell is restricted. Due to this fact, it’s essential for us to assemble a nanoformulation that may provide Fe3+ and cargo hydrophobic medicine. To assemble TPL@TFBF, we ready pure drug nanonuclear through a drug self-assembly technique, by merely dissolving the drug in DMSO answer after which including it dropwise to an aqueous answer beneath ultrasonication. This was adopted by the formation of a MOF shell layer by TA and Fe3+ coordination, and at last including BSA to make the nanoparticles extra steady. We first explored the affect of BSA dosing focus on the particle dimension and polydispersity index (PDI) (Fig. 2A), and located that the particle dimension and PDI have been minimized when the BSA dosing focus was 1 mg/mL, subsequently, we selected a BSA focus of 1 mg/mL for subsequent preparations. Then, we optimized the drug supply focus of TPL, and the outcomes confirmed that when the enter focus of TPL was 100 ~ 300 µg/mL, the particle dimension didn’t change considerably. Nevertheless, at 200 µg/mL TPL, the PDI was the smallest and the drug loading (LD%) (36.2%) and encapsulation effectivity (EE%) (81.4%) have been the best (Fig. 2B, C); subsequently, we selected a TPL enter focus of 200 µg/mL for the next TPL@TFBF nanoparticle preparation.
To endow the nanostructures with tumor-targeting properties, we modified BSA with FA, a ligand for the folate receptor that’s overexpressed on a number of varieties of tumor cells, together with B16F10 cells [37]. BSA-FA was synthesized by our earlier technique [38], and its profitable coupling was confirmed by UV spectroscopy (Fig. 2E). Utilizing BSA-FA as a stabilizer, the obtained TPL@TFBF had a dimension of roughly 200 nm and ξ potential of roughly − 17 mV (Fig. 2D, F), each of which have been similar to these of the TPL@TFB. Due to this fact, the impact of coupling FA onto BSA-modified TPL nanoparticles is small. As well as, we measured the particle dimension and zeta potential of TPL@TF utilizing a Malvern particle sizer (Fig. 2D, F and Extra file 1: Fig. S1) and located that the hydrodynamic particle dimension of TPL@TFB after BSA wrapping elevated barely from ~ 160 nm to ~ 200 nm, and the zeta potential decreased from roughly − 29 mV to roughly − 17 mV. Each of those outcomes show that BSA was successfully modified on the floor of TPL@TF. We used SDS–polyacrylamide gel electrophoresis to characterize the BSA within the pattern after TPL@TFBF depolymerization, and the info additional demonstrated the profitable inclusion of BSA (Extra file 1: Fig. S2). The TEM outcomes confirmed that the TPL@TFBF exhibited a typical spherical morphology (Fig. 2D) with a dimension of roughly 100 nm. That is barely lower than that measured by DLS as a result of DLS measures the hydrated particle dimension, and the growth of BSA in aqueous answer additionally will increase the particle dimension of TPL@TFBF. Elemental characterization of the TPL@TFBF was carried out utilizing EDS (Fig. 2G, H). The outcomes confirmed that Fe, a attribute ingredient in TA-Fe(III) MOFs, and S, a attribute ingredient in BSA, have been current within the NPs. These outcomes confirmed that the BSA-modified TA-Fe(III) MOFs have been efficiently ready. The nanoparticles exhibited wonderful colloidal stability in addition to long-term storage stability in numerous organic media because of the floor stabilization offered by BSA (Fig. 2I and Extra file 1: Fig. S3). Lastly, the LD% and EE% of Fe within the TPL@TFBF have been decided to be 3.28% and 29.52%, respectively, by ICP‒OES.
Results of A BSA dosing focus and B TPL dosing focus on nanoparticle dimension and PDI. C Impact of TPL focus on the LD% and EE% of TPL@TFBF. D TEM pictures and hydrodynamic dimensions of TPL@TFBF. Scale = 100 nm. E UV-vis spectra of FA, BSA, TPL, TA-Fe, BSA-FA, TPL@TFB and TPL@TFBF. F Zeta potentials of TPL@TF, TPL@TFB and TPL@TFBF. G The EDS evaluation of TPL@TFBF. (H) Floor ingredient composition of TPL@TFBF. I Stability of TPL@TFBF incubated in H2O, 10% FBS, RPMI-1640 cell medium (with 10% FBS) and PBS for 48 h
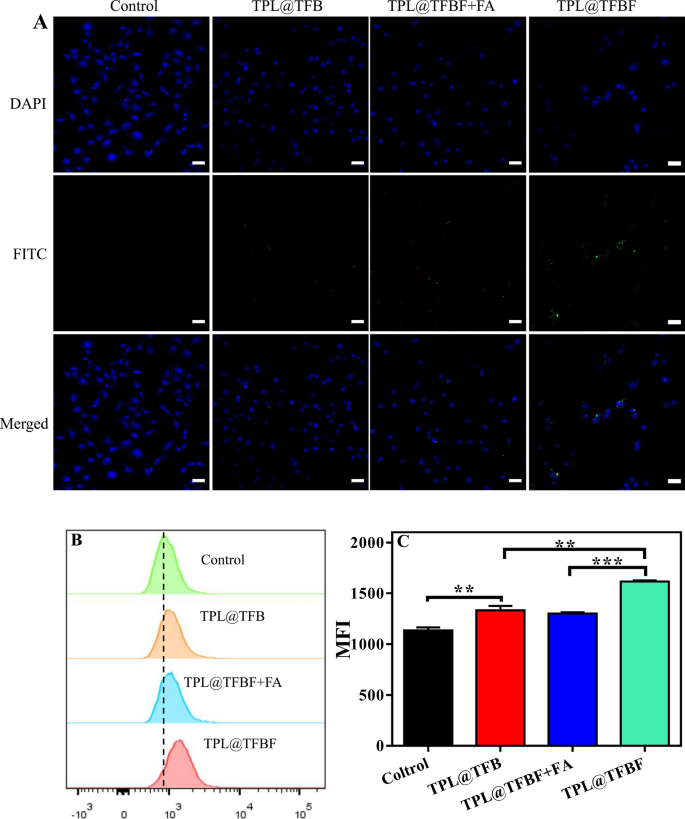
Tumor concentrating on in vitro
Subsequent, we studied the mobile uptake of TPL@TFBF through the use of B16F10 cells as a mannequin. To trace intracellular TPL@TFBF, in keeping with our earlier technique [38], BSA was labelled with inexperienced fluorescein isothiocyanate (FITC), and the nucleus was coloured blue with DAPI for localization. In contrast with the management group, the confocal laser scanning microscopy (CLSM) pictures confirmed that cells handled with TPL@TFB confirmed solely weak fluorescence, indicating much less uptake of TPL@TFB (Fig. 3A). This can be because of the cell’s rejection of the negatively charged nanoparticles. In distinction, the intracellular fluorescence was enhanced within the TPL@TFBF group. This apparently enhanced mobile uptake could also be attributed to folate receptor β (FR-β) overexpression on the membrane of B16F10 cells, selling the mobile uptake of TPL@TFBF by means of a selected interplay between FA and FR. To check this, we pretreated cells with free FA to bind to and saturate the folate receptor on the membrane. As anticipated, this triggered the intracellular fluorescence to be considerably lowered, additional demonstrating that FA modification can improve the uptake of nano-MOFs. For quantitative examination, we carried out circulate cytometry experiments (Fig. 3B) and quantified the fluorescence depth (Fig. 3C). In contrast with the TPL@TFB group, the fluorescence depth within the TPL@TFBF group was considerably enhanced, displaying the concentrating on capacity of those NPs. However, free FA-pretreated cells displayed elimination of this FA-mediated supply. General, the circulate cytometry outcomes have been consistent with the CLSM outcomes.
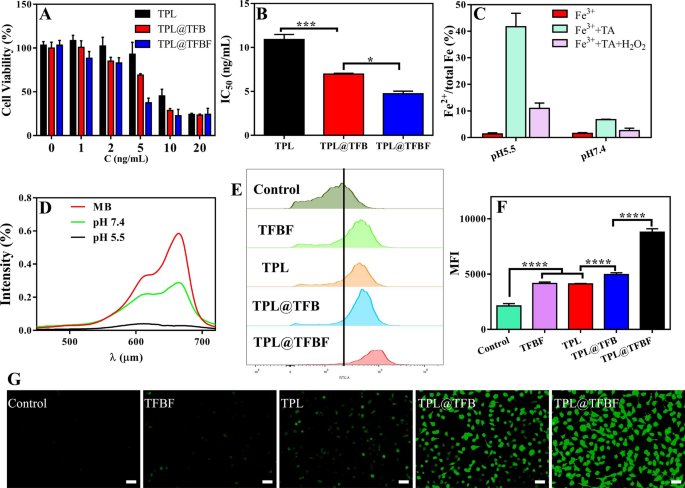
In vitro evaluation of TPL@TFBF
After demonstrating the tumor-targeted supply, we initially evaluated the in vitro toxicity of assorted brokers to B16F10 cells utilizing the CCK8 assay. After 48 h of therapy with completely different preparations containing completely different drug concentrations, cell viability decreased in a dose-dependent method (Fig. 4A), and at every focus, the TPL@TFBF exhibited stronger cytotoxicity than free TPL and TPL@TFB, resulting in vital decreases in IC50 values (Fig. 4B). This can be as a result of the nanoparticle-mediated supply of TPL and the cell inhibitory exercise of TPL have been considerably improved after it was integrated into nanoparticles. For the TPL@TFBF, FA modification enhanced the anti-tumor results by means of focused supply. ROS are key components that promote lipid peroxidation and play an essential function in numerous varieties of cell loss of life, amongst which hydroxyl radicals play an essential function in anticancer remedy [23]. The Fenton response mediated by iron ions can convert endogenous H2O2 into hydroxyl radicals (•OH), thereby growing intracellular ROS ranges, particularly Fe2+, which might set off a extra environment friendly Fenton response [39]. First, we verified whether or not Fe3+ could be lowered to Fe2+ by performing a 1,10-O-phenanthroline colorimetric assay earlier than TA-Fe3+ MOF software. As proven in Extra file 1: Fig. S4, the colour within the TFBF and TPL@TFBF teams didn’t change considerably after the addition of phenanthroline, and the conversion of Fe2+ was decided to be negligible (lower than 2%). This end result exhibits that TFBF and the TPL@TFBF have a weak capacity to scale back Fe3+ to Fe2+ earlier than software. Subsequent, we explored the power of TPL@TFBF to generate Fe2+ by TA discount of Fe3+ after cell entry. As proven in Fig. 4C, the conversion to Fe2+ was solely 6.9% after the addition of TA at pH 7.4, and because the pH of the system decreased, extra Fe3+ was lowered to Fe2+, reaching 40% at pH 5.5. The addition of H2O2 to the above system triggered the Fenton response mediated by Fe2+, and Fe2+ was transformed to Fe3+ once more. We additional verified the technology of free radicals through the use of the fading response of MB. As proven in Fig. 4D, free MB has robust ultraviolet absorption at 664 nm. The addition of Fe3+, TA and H2O2 results in the formation of •OH, which degrades MB and considerably reduces its ultraviolet absorption. Particularly, at pH 5.5, MB was utterly degraded. The above outcomes indicated that TA might cut back Fe3+ to Fe2+ beneath acidic circumstances, successfully triggering the Fenton response and changing H2O2 to •OH, confirming that the TPL@TFBF can induce the Fenton response in situ within the acidic tumor microenvironment. As well as, we measured intracellular ROS ranges utilizing the DCFH-DA probe. In contrast with the management group, the fluorescence depth of TFBF group was enhanced, additional demonstrating that TA reduces Fe3+ to Fe2+ after TFBF enters tumor cells, triggering the Fenton response and growing ROS manufacturing. The fluorescence depth was additionally enhanced within the TPL group in comparison with the management group, implying that TPL might additionally induce ROS manufacturing. Unexpectedly, the fluorescence depth of the TPL@TFB group was enhanced relative to that of the TFBF and TPL teams, implying that the Fe3+ getting into the cells might cooperate with TPL to extend the manufacturing of ROS. As well as, the strongest fluorescence depth was noticed within the TPL@TFBF group, which can be because of the focused supply mediated by FA, additional growing the extent of intracellular Fe3+ and synergistically amplifying the ROS sign (Fig. 4G). For quantitative evaluation, we additionally measured the intracellular fluorescence depth by circulate cytometry (Fig. 4E, F). The fluorescence depth of the TPL@TFBF group was 4 instances increased than that of the untreated group and considerably completely different in contrast with the opposite teams, which was in accordance with the pictures. This exhibits that the TPL@TFBF can considerably and synergistically amplify ROS manufacturing by means of the binding of Fe3+ and TPL.
A Viability of B16F10 cells handled with completely different preparations for 48 h. C (ng/mL) signifies the focus of TPL in every preparation. B IC50 values of various preparations. C Willpower of Fe2+ content material after completely different therapies. D Degradation of MB within the presence of H2O2 at pH 5.5 and seven.4. E ROS ranges in B16F10 cells detected by circulate cytometry after completely different therapies. F Quantification of the outcomes from (E). Knowledge are expressed because the imply ± SD (n = 4). ****P < 0.0001. G Fluorescence imaging of ROS in B16F10 cells handled with completely different preparations. Scale bar:50 μm
Verification of anti-tumor mechanism of motion
In abstract, there’s a synergistic amplification of ROS manufacturing by the mixture of Fe3+ and TPL, which led to a major enhancement within the efficacy of the TPL@TFBF. We subsequent examined the potential mechanisms. As everyone knows, Nrf2 has lengthy been thought-about an essential part of the antioxidant system that regulates the expression of antioxidant genes, thereby counteracting oxidative and pro-electrical stress [40]. As well as, a sequence of redox-related genes focused by Nrf2 are key mediators of ferroptosis. For instance, the inhibition of GPX4-induced ferroptosis may very well be reversed by overexpressing Nrf2 [41]. Nevertheless, TPL can immediately inhibit the expression of Nrf2 and intrude with de novo synthesis of glutathione [28], thereby growing ferroptosis sensitivity and producing antitumor results. However, the Fe3+ mediated Fenton response can elevate intracellular ROS ranges to induce ferroptosis. As well as, manipulating the content material of intracellular iron was discovered to induce pyroptosis [23]. Due to this fact, the mixture of TPL and Fe3+ could improve the therapeutic impact by synergistically amplifying ROS manufacturing, thereby inflicting simultaneous ferroptosis and pyroptosis. We subsequent examined whether or not TPL@TFBF might induce each ferroptosis and pyroptosis.
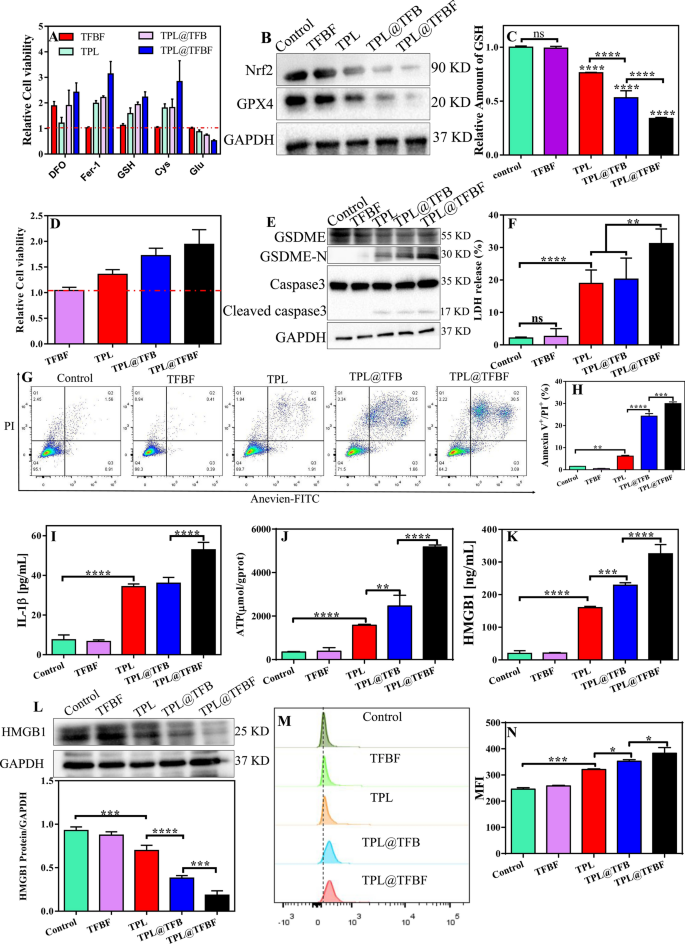
First, B16F10 cells have been handled with numerous ferroptosis inhibitors/promoters together with numerous preparations, and the relative cytotoxicity was calculated by comparability with the group not handled with ferroptosis inhibitors/promoters. As proven in Fig. 5A, the ferroptosis inhibitor Fer-1 alleviated the cytotoxicity of the TPL@TFBF. Equally, the iron chelator DFO, the ferroptosis inhibitor Cys, and the ferroptosis detoxifier GSH attenuated the cytotoxicity of TPL@TFBF. In distinction, Glu, as a ferroptosis inducer, achieved the other impact by inhibiting Cys uptake. General, all these outcomes show that the constructed of TPL@TFBF can successfully promote ferroptosis. To additional discover the mechanism by which TPL@TFBF induces ferroptosis, the expression ranges of Nrf2 and GPX4 have been detected by WB. GPX4 is a marker of ferroptosis. In contrast with that within the management and TFBF teams, the expression of Nrf2 and GPX4 was downregulated in B16F10 cells handled with TPL, TPL@TFB and TPL@TFBF, and notably, their expression was the bottom within the TPL@TFBF group (Fig. 5B), suggesting that the codelivery of TPL and Fe3+ might considerably cut back the expression of Nrf2 and GPX4. Quantification of the protein ranges additional confirmed this vital change (Extra file 1: Fig. S5). Correspondingly, intracellular GSH ranges confirmed the identical downwards pattern (Fig. 5C). Since GPX4 requires the help of GSH to take part in lipid peroxidation, the downregulation of GSH induced by TPL can promote the downregulation of GPX4 to induce ferroptosis. By verifying these two key components required for ferroptosis (GSH discount and GPX4 downregulation), it was confirmed that the TPL@TFBF might induce ferroptosis.
TPL has been discovered to induce GSDME-dependent pyroptosis [42], and iron-activated ROS have beforehand been reported to induce pyroptosis through the Tom20-Bax-caspase-GSDME pathway [24]. Due to this fact, additional analysis was wanted to find out whether or not GSDME-dependent pyroptosis additionally contributes to TPL@TFBF-induced cell loss of life. GSDME-dependent pyroptosis is principally activated by caspase-3. After activation, the GSDME protein hydrolyses and releases N fragments, which assemble to kind pores on the cell membrane. The cell swelling and plasma membrane rupture end result within the destruction of membrane integrity and the discharge of mobile contents [43, 44]. To find out whether or not TPL@TFBF induced pyroptosis through the GSDME pathway, we handled B16F10 cells with DEVD. As proven in Fig. 5D, the cytotoxicities of TPL, TPL@TFB and TPL@TFBF have been considerably lowered by the addition of DEVD, and the TPL@TFBF confirmed a stronger impact, suggesting that TPL@TFBF-induced cell loss of life could rely on the caspase-3-mediated pathway. To additional discover the pyroptosis mechanism of TPL@TFBF therapy of B16F10 cells, we examined the degrees of pyroptosis-associated protein in B16F10 cells utilizing WB. As proven in Fig. 5E, cleaved caspase-3 and GSDME-N expression have been elevated within the TPL, TPL@TFB, and TPL@TFBF teams, and GSDME-N was extra strongly upregulated within the TPL@TFBF group than within the different teams. These outcomes indicated that free TPL might induce pyroptosis mediated by caspase-3, and the addition of Fe3+ might higher induce pyroptosis. This phenomenon was additional confirmed by semiquantitative evaluation (Extra file 1: Fig. S6), which confirmed that the expression stage of GSDME-N within the TPL@TFB group was 1.5 instances that within the TPL group and that within the TPL@TFBF group was 1.5 instances that within the TPL@TFB group. These outcomes recommend that TPL@TFBF can activate caspase-3 to cleave GSDME into GSDME-N and additional induce pyroptosis. Subsequently, we evaluated the discharge of lactate dehydrogenase (LDH, an indicator of pyroptosis) and IL-1β into the cell supernatant to look at the leakage of intracellular contents. The discharge of LDH and IL-1β into the supernatant of TPL@TFBF-treated cells was considerably increased than that within the different therapy teams (Fig. 5F and I). To additional show the power of the TPL@TFBF to induce pyroptosis, we used Annexin V-FITC/PI double staining evaluation as reported within the literature [45,46,47]. As proven in Fig. 5G, H and Extra file 1: Fig. S7, B16F10 cells underwent vital pyroptosis after TPL@TFBF therapy. Furthermore, there have been only a few early apoptotic cells, which might rule out the incidence of apoptosis. Thus, TPL@TFBF enhanced caspase-3 mediated GSDME cleavage and elevated the discharge of IL-1β and LDH, confirming that TPL@TFBF is an effective inducer of pyroptosis.
Induction of ICD
Earlier research have proven that each pyroptosis and ferroptosis could result in immunogenic cell loss of life (ICD), so there is a chance to make use of this mix with immunotherapy for synergistic results [48,49,50]. To discover this impact, we subsequent studied the DAMPs launched from dying cells, together with HMGB1, ATP and CRT, that are essential options of ICD. We first examined the discharge of ATP and HMGB1 into the cell supernatants, and extracellular ATP and HMGB1 had been launched within the TPL, TPL@TFB and TPL@TFBF teams as compared with the management and TFBF teams, particularly in TPL@TFBF teams (Fig. 5J, Ok). We additionally detected intracellular HMGB1 ranges by WB. In contrast with the management and TFBF teams, TPL, TPL@TFB and TPL@TFBF have been all efficient in lowering HMGB1 focus, and the discount impact of TPL@TFBF was extra vital (Fig. 5L). We then examined CRT publicity by circulate cytometry, and the outcomes confirmed that TPL@TFBF considerably elevated CRT publicity (Fig. 5M); furthermore, quantitative evaluation confirmed that the CRT publicity induced by the TPL@TFBF was vital in contrast with that within the different therapy teams (Fig. 5N). These outcomes recommend that the power of TPL@TFBF to induce immunogenicity is enhanced to some extent by FA modification on account of tumor concentrating on exercise. General, cells skilled ICDs after therapy with TPL@TFBF, which may very well be attributed to pyroptosis and ferroptosis cytotoxicity induced by nano-MOFs.
A Relative cell viability of B16F10 cells after incubation in several preparation teams (TFBF, TPL, TPL@TFB and TPL@TFBF with and with out numerous compounds). B Expression of Nrf2 and GPX4 proteins after completely different therapies. C Relative GSH ranges in B16F10 cells after completely different preparations. Knowledge are expressed because the imply ± SD (n = 3). ns, not vital, ****P < 0.0001. D Relative cell viability (in comparison with the group with out inhibitor therapy) of B16F10 cells after completely different therapies within the presence of DEVD. E Expression of GSDME, GSDME-N and cleaved-caspase-3 proteins after completely different therapies. F LDH launch from B16F10 cells after completely different therapies. Knowledge are expressed because the imply ± SD (n = 3). ns, not vital, **P < 0.01, ****P < 0.0001. G Annexin V/PI double staining evaluation of B16F10 cells handled with completely different preparations. H Annexin V+/PI+ quantification of (G). Knowledge are expressed because the imply ± SD (n = 3). **P < 0.01, ***P < 0.001, ****P < 0.0001. Launch of IL-1β (I), ATP (J) and (Ok) HMGB1 from B16F10 cells after therapy with completely different preparations. L Expression of the HMGB1 protein in B16F10 cells after completely different therapies. Knowledge are offered because the imply ± SD (n = 3). ***P < 0.001, ****P < 0.0001. M Movement cytometric outcomes of CRT publicity from B16F10 cells handled with completely different brokers and (N) quantitative common fluorescence depth. Knowledge are expressed because the imply ± SD (n = 3). *P < 0.05, ***P < 0.001
In vivo efficiency of nano-MOFs
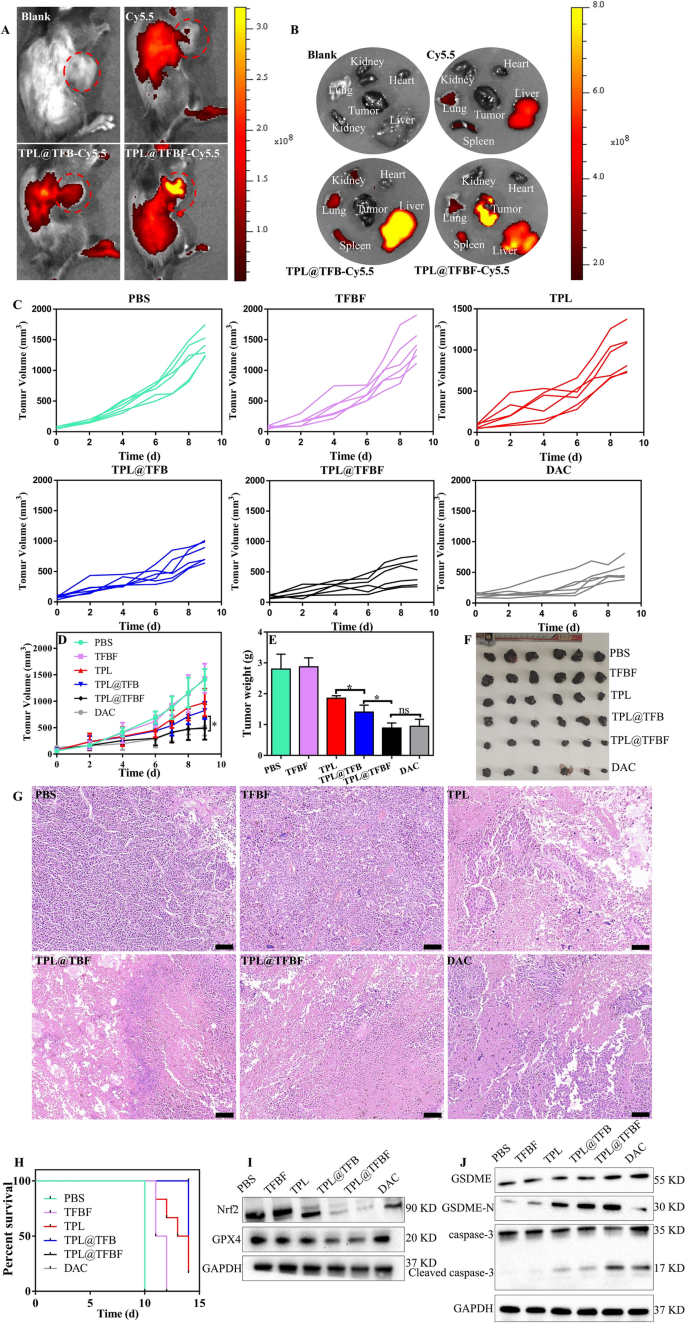
As we demonstrated, TPL@TFBF can induce ferroptosis and pyroptosis in vitro. Due to this fact, we hypothesized that TPL@TFBF may inhibit tumor development in vivo. Subsequent, we utilized a B16F10 tumor-bearing mouse mannequin to characterize the efficiency of TPL@TFBF in vivo. Blood compatibility assessments have been carried out earlier than the nanoparticles have been injected intravenously. At concentrations as excessive as 200 µg/ml, hemolysis charges of lower than 2% have been noticed after with TPL@TFBF (Extra file 1: Fig. S8), indicating their excessive biosafety after intravenous administration. To review the in vivo biodistribution, TPL@TFBF was labelled with Cy5.5. After 24 h of nanoparticle injection, the fluorescence of the nanoparticles was a lot stronger than that of free Cy5.5, which can be attributed to the longer circulation half-life of the nanosystem. It’s price noting that vital fluorescence from the nanoparticles was noticed in tumor tissue, indicating tumor accumulation by means of the EPR impact, particularly for the FA-modified nanoparticles, indicating the lively concentrating on functionality of the nanoparticles in vivo (Fig. 6A). As well as, we extracted the main organs and tumor tissues of the mice for ex vivo fluorescence imaging (Fig. 6B) and noticed vital fluorescence from TPL@TFBF-Cy5.5 within the tumor tissues, which was in keeping with the in vivo outcomes. General, our nano-MOFs can actively goal tumors to ship medicine, which will likely be helpful for bettering therapeutic efficacy and minimizing nonspecific unintended effects.
As a way to consider the anti-tumor results in vivo, tumor-bearing mice have been randomly divided into 6 teams and handled with completely different formulations. The tumor dimension was measured to observe the dynamic efficacy. The tumor quantity elevated quickly throughout therapy within the PBS and TFBF teams, indicating that TFBF had no tumor suppressive impact. In contrast with the PBS group, the free TPL group had reasonable tumor development inhibition (~ 30.97%), and the TPL@TFB group confirmed higher tumor management than TPL (~ 41.74%), suggesting a synergistic impact from TPL and Fe3+ in vivo. The tumor suppression impact of TPL@TFBF (~ 65.58%) was enhanced to some extent by FA modification, virtually reaching that of the optimistic drug DAC (~ 63.47%), in all probability on account of tumor concentrating on (Fig. 6C, D). Tumor tissues have been extracted after therapy for direct comparability (Fig. 6E, F), and the tumor weights and corresponding tumor pictures have been in keeping with the general pattern of tumor quantity. We additional examined the tumor tissue by H&E staining to guage the therapeutic impact. In Fig. 6G, we noticed vital cell necrosis and nucleopaenia within the TPL@TFBF and DAC teams. As well as, we constructed survival curves for every group (Fig. 6H), and the survival fee was 16.67% within the free TPL group and 83% within the TPL@TFBF group after 14 days, indicating that the TPL@TFBF considerably extended the survival of mice in contrast with free TPL. Thus, the above outcomes recommend that TPL@TFBF can enhance the effectivity of melanoma therapy and alleviate unintended effects.
To additional examine the tumor suppression mechanism of motion, we assessed the expression ranges of GPX4, Nrf2, cleaved caspase-3 and GSDME-N within the tumor area (Fig. 6I, J). According to the outcomes of the in vitro evaluation, GPX4 and Nrf2 on the TPL@TFBF-treated mouse tumor websites have been considerably downregulated, and cleaved caspase-3 and GSDME-N have been considerably upregulated in contrast with the TPL and TPL@TFB teams, indicating that TPL@TFBF might exert anti-tumor results by inhibiting Nrf2 expression and elevating intracellular ROS, thereby inducing ferroptosis and pyroptosis.
Furthermore, the protection of the nanosystem was evaluated. There was little change within the weights of the mice throughout therapy (Extra file 1: Fig. S9), indicating no acute toxicity. Evaluation of the serum biochemical indices after therapy confirmed that liver operate and renal operate have been inside the regular vary (Extra file 1: Fig. S10), indicating that the nano-MOFs had no hepatotoxicity or renal toxicity. Additional H&E staining evaluation of main organs confirmed no pathological modifications (Extra file 1: Fig. S11). As well as, to find out whether or not the discharge of assorted inflammatory components from ferroptosis and pyroptosis might trigger cytokine storm syndrome, we additionally measured the modifications in serum TNF-α and IL-6 ranges, and the modifications after numerous therapies have been negligible (Extra file 1: Fig. S12). General, our nano-MOF is a platform with excessive biosafety.
Fluorescence pictures of tumor-bearing mice in vivo A and in vitro B after completely different therapies. C–D The tumor development curves of mice publish completely different therapies. E Weight, F histograms, and G H&E staining of tumors in every group on the finish of therapy. Scale bar, 100 μm. Knowledge are expressed because the imply ± SD (n = 6). ns, not vital, *P < 0.05. H Survival charges of mice handled with every preparation. I The expression ranges of Nrf2 and GPX4 in several teams of tumor tissues have been detected by WB. J Expression ranges of GSDME, GSDME-N, cleaved caspase-3 in tumor tissues after therapy
Antitumor immunity induced by TPL@TFBF
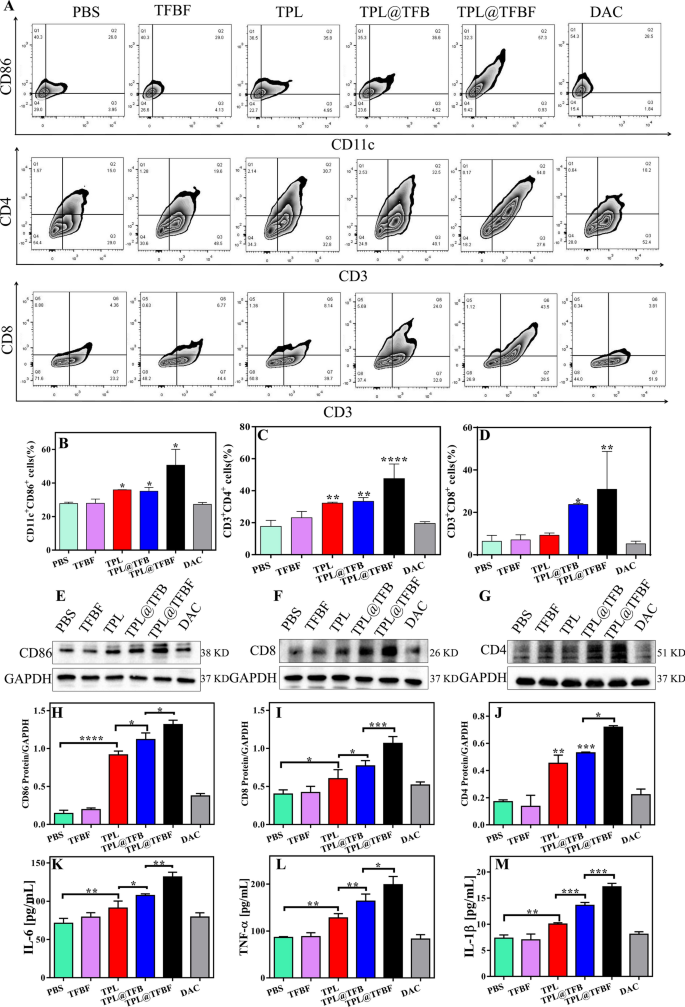
To review the in vivo immune impact, we eliminated tumors after euthanasia of the mice and detected the proportion of immune cells within the tumor tissue by circulate cytometry. First, mature dendritic cells (mDCs) and tumor-infiltrating T cells have been detected by circulate cytometry. The outcomes confirmed that in contrast with PBS or TFBF, the proportion of activated DCs (CD86+CD11c+) in TPL@TFBF tumor tissues elevated from 36.6 to 68.85%, indicating an elevated stage of DCs maturation after TPL@TFBF therapy (Fig. 7A, B and Extra file 1: Fig. S13). This was because of the simultaneous incidence of ferroptosis and pyroptosis after therapy with the TPL@TFBF, which results in a stronger immune impact, thus amplifying the maturation of DCs. After DCs maturation, antigens are offered to T cells to provoke T-cell cloning and proliferation. We monitored the ratio of cytotoxic T cells (CD8+) and helper T cells (CD4+) (Fig. 7A, C and D) and located that tumor infiltration of cytotoxic T lymphocytes (ctls, CD3+CD8+ T cells) and helper T cells (Ths, CD3+CD4+ T cells) the TPL@TFBF group was considerably increased than that in different teams. These outcomes recommend that TPL@TFBF therapy can promote T-cell clonal growth. Then, the expression ranges of immune-related proteins in mouse tumor tissues have been investigated by WB (Fig. 7E, J). After TPL therapy, the expressions of CD86, CD4 and CD8 in mouse tumor tissues have been observably up-regulated. The expression of the above proteins was additional up-regulated after therapy with TPL@TFB, and once more after therapy with TPL@TFBF, indicating the synergistic immune activation impact of TPL and Fe3+. The expression of the above proteins was additional investigated by immunofluorescence, and the outcomes have been principally in keeping with these of WB, indicating that TPL@TFBF therapy initiated an adaptive immune response (Extra file 1: Fig. S14). T cells are activated and secrete cytokines reminiscent of tumor necrosis issue α (TNF-α) and interleukin 6 (IL-6), which immediately kill tumor cells. The degrees of TNF-α and IL-10 in mouse tumor tissues have been considerably elevated after therapy with TPL@TFBF (Fig. 7Ok and L), which additionally indicated the efficient activation of anti-tumor immunity. As well as, IL-1β ranges of pyroptosis related inflammasomes have been elevated, suggesting pyroptosis (Fig. 7M). Collectively, these in vivo experimental outcomes present that TPL@TFBF may cause ferroptosis and pyroptosis in most cancers cells, improve immunogenicity, promote DCs activation, and set off a T-cell-dependent immune response for wonderful anti-tumor efficacy and environment friendly stimulation of adaptive anti-tumor immunity.
A Movement cytometry evaluation of CD11c+CD86+ cells, CD3+CD4+ cells and CD3+CD8+ cells in tumor tissues from mice in every group after therapy with completely different formulations. B–D Quantification of the outcomes from (A). E–G Expression ranges of CD86, CD8 and CD4 in tumor tissues from completely different teams. H–J Quantification of the outcomes from (E–G). The degrees of Ok IL-6, L TNF-α and M IL-1β in tumor tissues from mice in several teams after completely different therapies. Knowledge are expressed because the imply ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
TPL@TFBF inhibits tumor metastasis, and its mixture with aPD-L1 enhances immunotherapy
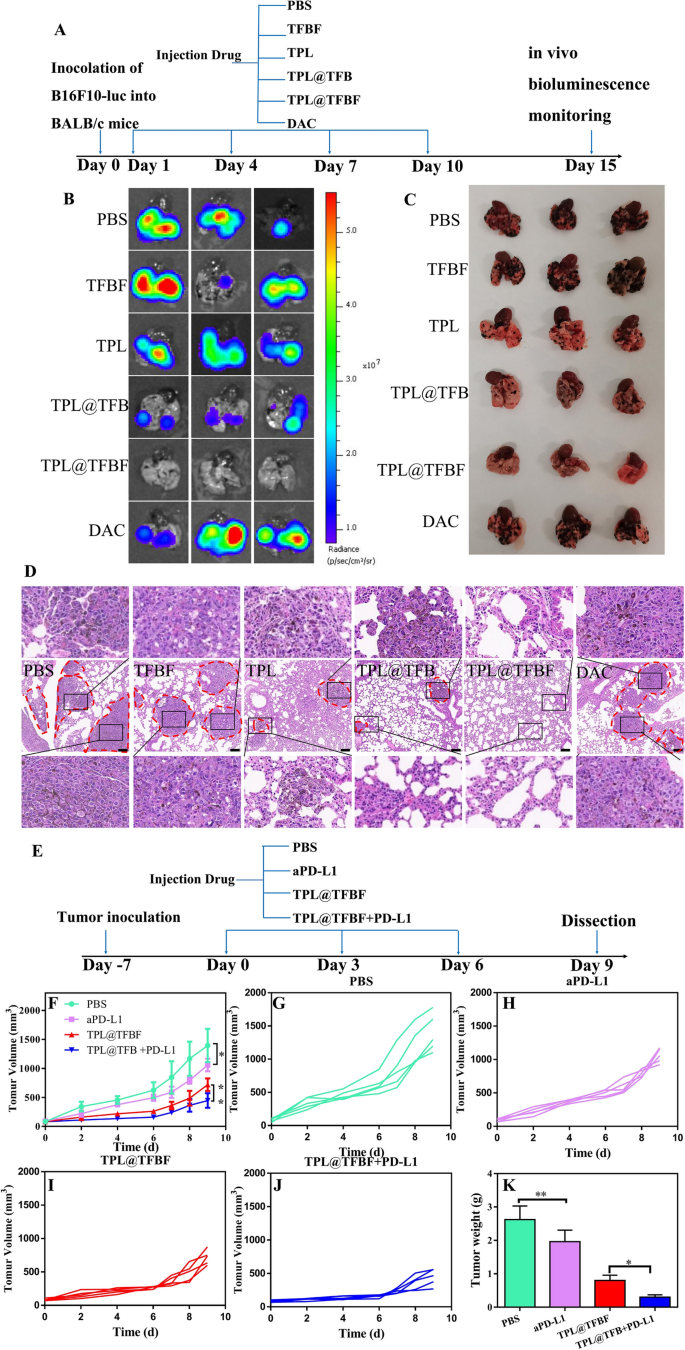
The primary explanation for loss of life in sufferers with medical melanoma is because of their susceptibility to lung metastasis, which can be a significant problem in most cancers therapy. Fortuitously, TPL has been proven to inhibit the switch of B16F10 cells to the lungs and spleens of mice. As well as, immunotherapy has the potential to inhibit metastasis. As indicated above, our nano-MOFs produce an excellent immune response. We constructed a lung metastatic tumor mannequin by intravenously injecting B16F10-luc cells and evaluated the power of TPL@TFBF to inhibit tumor metastasis. After completely different therapies, in vitro bioluminescence imaging was carried out utilizing fluorescein because the substrate (Fig. 8A). The lung tissue from the PBS group confirmed a robust fluorescence sign, indicating the profitable development of the mouse lung metastasis mannequin. There was no vital change within the fluorescence sign within the TFBF and DAC teams, and the results of TFBF and DAC on lung metastasis have been apparently restricted. After therapy with TPL and TPL@TFB, the fluorescence sign in mouse lung tissue have been considerably lowered, indicating their inhibition of lung metastasis. After therapy with TPL@TFBF, there was virtually no fluorescence sign within the lung tissue, indicating that metastasis activation was utterly suppressed, which may very well be attributed to the robust immune activation produced by the TPL@TFBF (Fig. 8B).
Subsequent, lung tissues have been collected for metastatic web site evaluation. A lot of pulmonary metastatic nodules with melanoma cell aggregates have been noticed within the PBS group, whereas no apparent metastatic nodules have been noticed within the TPL@TFBF group, confirming that TPL@TFBF can successfully inhibit pulmonary metastasis (Fig. 8C). To verify this, we carried out additional H&E staining evaluations on every sort of lung tissue, and the lung metastatic are circled with pink dashed circles in Fig. 8D. Giant tumor metastases have been seen within the PBS group, TFBF group and DAC group, just a few smaller metastases remained within the TPL group and TPL@TFB group, and no apparent metastases have been seen within the TPL@TFBF group, confirming that TPL itself might inhibit lung metastasis, however TPL@TFBF may additional inhibit lung metastasis on account of activation of the immune response.
We additional used the nanosystem together with the ICB PD-L1 antibody (aPD-L1) for melanoma for tumor co-immunotherapy. Institution of the mouse mannequin and the great therapy protocol are proven in Fig. 8E. Notably, the only agent aPD-L1 confirmed solely marginal tumor suppression. TPL@TFBF was superior to aPD-L1 on account of its robust immunomodulatory impact, which have been markedly enhanced when utilized in mixture with aPD-L1 (Fig. 8F, Ok and Extra file 1: Fig. S15). On this mixture, TPL@TFBF promotes DCs activation and T-cell infiltration, whereas aPD-L1 prompts T cells to assault tumor cells, producing a synergistic anti-tumor impact.
A Schematic diagram of the completely different B16F10-luc lung metastatic tumor therapies. B Bioluminescence imaging, C direct view and D H&E staining of lung tissue from mice with metastases after intervention with completely different preparations. E Schematic diagram of the dosing routine for mixture aPD-L1 therapy. F–J Tumor development curves throughout completely different therapies. Ok Tumor weights after completely different therapies. Knowledge are expressed because the imply ± SD (n = 5). *P < 0.05, **P < 0.01