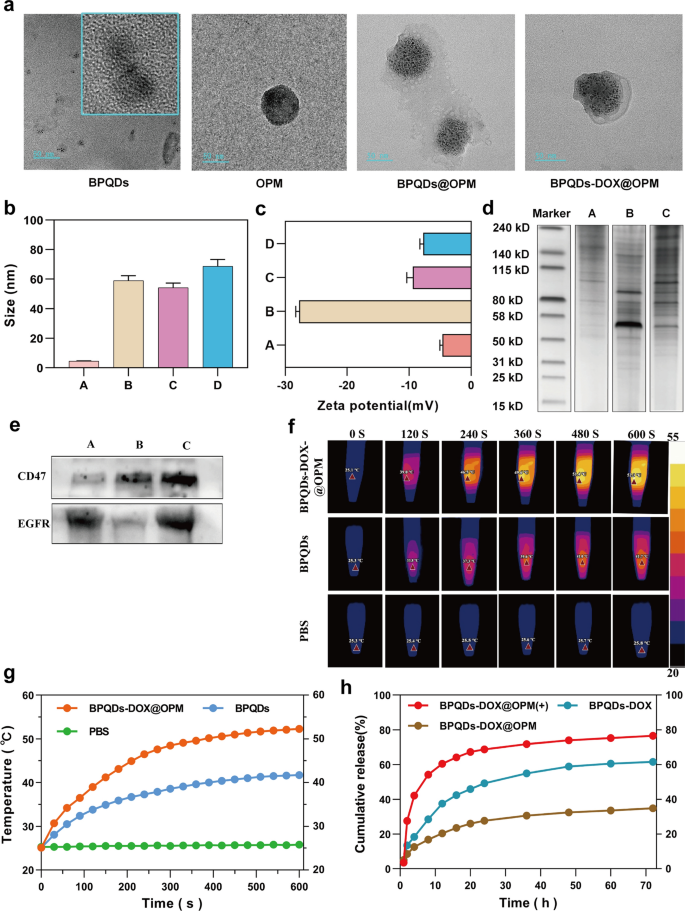
Examination of OPM properties on BPQDs-DOX@OPM
The mobile hybrid membrane-encapsulated nanoparticles encompass two most important components: nanocarrier core and floor cell membrane shell construction [32]. As a result of the hybrid membrane can retain glycoproteins, lipids, and proteins on your entire cell membrane floor, it will possibly endow the nanoparticles with capabilities and properties inherent to the supply cell. For instance, CD47 on the platelet floor can work together with immune cells to inhibit immune clearance [27], and most cancers cells will be homotypically focused [28]. To look at the proteins on BPQDs-DOX@OPM, we examined the hybrid membrane complete protein and signature protein expression by SDS-PAGE gel electrophoresis and Western blot, respectively. The SDS-PAGE gel electrophoresis outcomes are proven in Fig. 2d; they point out that the BPQDs-DOX@OPM group has each OCM and PM on the protein. The Western blot outcomes are proven in Fig. 2e; they point out that the OCM, PM, and BPQDs-DOX@OPM teams confirmed the expression of the signature protein EGFR, signature protein CD47, and each EGFR and CD47, respectively. The outcomes of SDS- PAGE gel electrophoresis and Western blot indicated that BPQDs-DOX@OPM contained an OPM heterodimeric membrane with full protein expression.
BPQDs-DOX@OPM characterization. a Transmission electron micrographs of BPQDs, OPM, BPQDs@OPM, and BPQDs-DOX@OPM (measurement: 50 nm). b, c Particle measurement and zeta potential of every group (A: BPQDs, B: OPM, C: BPQDs@OPM, D: BPQDs-DOX@OPM). d SDS-PAGE gel electrophoresis consequence (A: OCM, B: PM, C: BPQDs-DOX@OPM). e Western blot evaluation consequence (A: OCM, B: PM, C: BPQDs-DOX@OPM). f, g Photothermal efficiency and temperature rise curve of BPQDs-DOX@OPM. h DOX launch traits of BPQDs-DOX@OPM
BPQDs-DOX@OPM-like traits
In cell membrane-mimetic encapsulation of small-sized nanocarriers, the dimensions of the cell membrane-modified nanocarriers is normally near that of the cell membrane, and the floor potential of the drug supply system is near that of the cell membrane [33, 34]. The TEM outcomes (Fig. 2a) confirmed that the BPQDs in addition to BPQDs@OPM hybrid membrane vesicles had a rounded morphology and good dispersion, and OPM had a shell construction that fully encapsulated a number of BPQDs. The TEM outcomes (Fig. 2b) confirmed that the particle sizes of the BPQDs, OPM, BPQDs@OPM, and BPQDs-DOX@OPM teams have been 4.53 ± 1.36 nm, 58.96 ± 13.65 nm, 54.22 ± 17.69 nm, and 68.80 ± 23.78 nm, respectively; the particle measurement of BPQDs-DOX@OPM was near that of OPM vesicles. The zeta potentials (Fig. 2c) of BPQDs, OPM, BPQDs@OPM, and BPQDs-DOX@OPM as measured by DLS have been − 18.07 ± 0.46 mv, − 9.43 ± 0.78 mv, − 4.64 ± 0.34 mv, and − 7.70 ± 0.46 mv, respectively; the zeta potential of BPQDs-DOX@OPM was near that of OPM.
Photothermal conversion efficiency of BPQDs-DOX@OPM
Zero-dimensional BPQDs with a 0–10 nm measurement exhibit a large absorption vary all through the seen area. Consequently, they’ve good photothermal conversion effectivity [35]. Nonetheless, BPQDs are inclined to oxidative degradation in an setting containing oxygen and water; biofilm encapsulation can isolate the interior BPQDs from oxygen and water to enhance their stability [36]. As proven in Fig. 2f and g, After exposing the BPQDs and BPQDs-DOX@OPM to the air setting for 4 days, every set of options was irradiated with near-infrared mild (808 nm, 1.0 W/cm2) for 10 min. The temperature of the PBS group didn’t change considerably. Against this, temperatures of the BPQDs and BPQDs-DOX@OPM teams elevated quickly and reached 41.7 °C and 52.3 °C, respectively, after 600 s. Additional, the ultimate temperature of the BPQDs-DOX@OPM was increased than each the BPQDs and the tissue cell deadly temperature of 48 °C [11].
BPQDs-DOX@OPM drug sluggish launch curve
The calculated drug loading and encapsulation effectivity have been 36.2% and 73.4%, respectively, primarily based on the linear regression equation of the DOX customary curve. As proven in Fig. 2h, the 72-h launch of DOX from BPQDs-DOX@OPM and BPQDs-DOX was 34.88% and 61.53%, respectively; this indicated that the OPM hybrid movie encapsulation system might decelerate the discharge of DOX and assist lengthen the half-life of BPQDs-DOX@OPM within the blood circulation system. After irradiation with 808-nm NIR mild, owing to photostimulation-responsive launch, the 72-h DOX launch of the BPQDs-DOX@OPM(+) group was 76.04%; this was considerably increased than that of the BPQDs-DOX@OPM group with out mild irradiation.
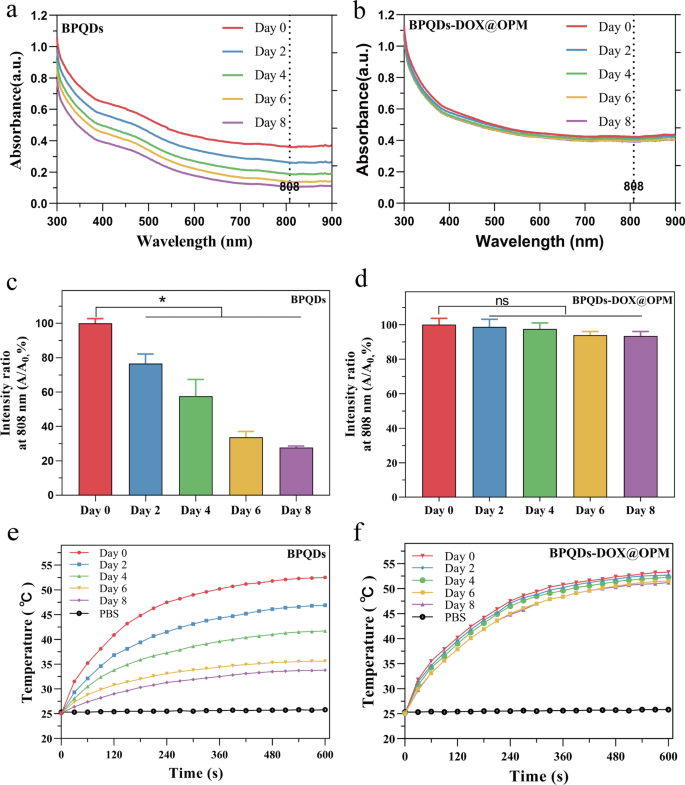
In vitro stability evaluation of BPQDs-DOX@OPM
Zero-dimensional BPQDs, with sizes starting from 0 to 10 nm, show broad absorption throughout the seen area, indicating favorable photothermal conversion effectivity. Nonetheless, BPQDs are inclined to oxidative degradation within the presence of oxygen and water. Biofilm encapsulation know-how was employed to isolate the interior BPQDs from oxygen and water to reinforce their stability. To judge the improved stability conferred by OPM encapsulation, BPQDs and BPQDs-DOX@OPM, each with constant concentrations, have been dispersed in water and uncovered to air for 8 days. Optical property measurements have been carried out at particular intervals (0, 2, 4, 6, and eight days).
Determine 3a, b illustrates the everyday broad absorption bands within the UV and NIR areas exhibited by each BPQDs and BPQDs-DOX@OPM. Nonetheless, the absorbance depth of BPQDs in water decreased over time, whereas no important lower in absorbance depth was noticed for BPQDs-DOX@OPM. The proportion change in absorbance depth at 808 nm for BPQDs and BPQDs-DOX@OPM is depicted in Fig. 3c, d, respectively. After 2 days, the absorbance depth of BPQDs decreased by 23.4% ± 4.47%, and after 8 days, it decreased by 72.3% ± 0.71%. This lower was attributed to the degradation of naked BPQDs, leading to diminished absorbance. In distinction, the absorbance of BPQDs-DOX@OPM remained extra secure, with solely a 6.57% ± 2.14% lower after 8 days. This enhanced stability will be attributed to the shell construction of OPM, which reduces the affect of oxygen and water on BPQDs, thereby bettering the soundness of BPQDs-DOX@OPM underneath environmental circumstances.
UV–Vis absorption spectra of BPQDs and BPQDs-DOX@OPM at totally different time factors and the corresponding temperature rise curve. a Absorption spectra of BPQDs at 0, 2, 4, 6, and eight days; b absorption spectra of BPQDs-DOX@OPM at 0, 2, 4, 6, and eight days; c statistical evaluation of the absorbance of BPQDs at 808 nm at totally different time factors; d statistical evaluation of the absorbance of BPQDs-DOX@OPM at 808 nm at totally different time factors; e temperature rise curves of BPQDs on days 0, 2, 4, 6, and eight days; f temperature rise curves of BPQDs-DOX@OPM on days 0, 2, 4, 6, and eight days
Determine 3e presents the temperature rise curve of BPQDs, displaying a lower over time because of the unstable nature and simple oxidative degradation of BPQDs. Conversely, Fig. 3f demonstrates that the temperature rise curve of BPQDs-DOX@OPM doesn’t exhibit a major lower over time. This remark aligns with the outcomes obtained from the UV–vis absorption spectra of BPQDs-DOX@OPM, confirming that the OPM shell construction enhances the soundness of BPQDs underneath environmental circumstances.
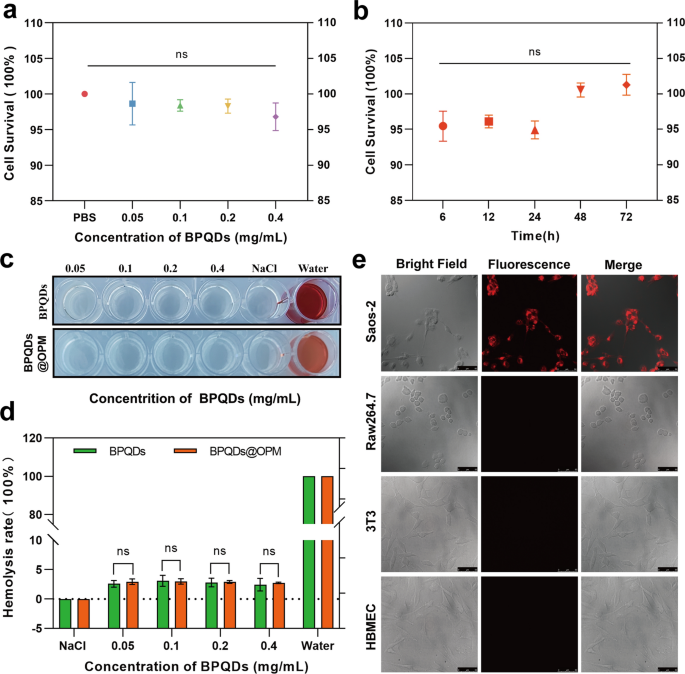
In vitro biocompatibility of BPQDs@OPM drug supply system
Cytotoxicity of BPQDs@OPM drug supply system on Saos-2 cells
The in vitro biocompatibility evaluation of nanocarriers relating to tissue cytotoxicity and hemocompatibility is taken into account essentially the most dependable and easiest technical software in biosafety evaluation [37]. Finding out the dose- and time-dependent cytotoxicity of nanocarriers is without doubt one of the most typical strategies to detect cytotoxicity in vitro [38]. Subsequently, as proven in Fig. 4a, Saos-2 was plated for 12 h after BPQDs@OPM handled cells. The outcomes of the CCK-8 cell proliferation exercise assay confirmed that the relative cell survival fee of every group was maintained above 95% after coincubation of BPQDs@OPM with cells at totally different concentrations for twenty-four h, and the distinction was not statistically important. As proven in Fig. 4b, after 12 h of Saos-2 cell plating, the BPQDs@OPM system was coincubated with Saos-2 cells for six, 12, 24, 48, and 72 h, and the CCK-8 cells have been assayed for relative cell viability. The assay outcomes confirmed that the cell proliferation exercise of the BPQDs@OPM drug supply system was maintained above 95% at totally different intervals of coincubation with Saos-2 cells, and the variations weren’t statistically totally different. The dose- and time-effect relationships of BPQDs@OPM each indicated its excessive biocompatibility.
BPQDs-DOX@OPM in vitro efficiency research. a Cell viability of BPQDs@OPM with totally different BPQD concentrations after coincubation with Saos-2 for twenty-four h. b Cell viability of BPQDs@OPM with BPQD focus of 0.2 mg/mL after coincubation with Saos-2 cells for various intervals. c Pictures of supernatants of BPQDs and BPQDs-DOX@OPM hemolysis experiments. d Calculation of hemolysis charges of BPQDs and BPQDs-DOX@OPM. e DID fluorescence-labeled BPQDs-DOX@OPM after coincubation with Saos-2, 3T3, and HBMEC, and RAW264.7 for 4 h. Laser confocal microscopy imaging (measurement: 50 μm)
Hemocompatibility of BPQDs@OPM drug supply system
Chemotherapeutic drug carriers are largely delivered to the tumor web site by way of blood circulation. Investigating their hemocompatibility will help additional perceive the protection and efficacy of drug supply techniques [39, 40]. Subsequently, the erythrocyte hemolysis assay was chosen to confirm the BPQDs@OPM hemocompatibility on this research. As proven in Fig. 4c and d, after coincubation of every remedy group with the erythrocyte suspension for 1 h, the erythrocyte hemolysis charges of BPQDs@OPM teams with BPQD concentrations of 0.05, 0.1, 0.2, and 0.4 mg/mL have been under 3%. The BPQDs group with 0.1 mg/mL BPQD focus confirmed the very best erythrocyte hemolysis fee of three.10 ± 0.75%. The hemolysis fee of BPQDs and BPQDs-DOX in all focus teams was lower than 5%, in accordance with the required hemolysis fee for organic supplies; this indicated that each BPQDs and BPQDs@OPM had good hemocompatibility.
In vitro focusing on research of BPQDs-DOX@OPM
As proven in Fig. 4e, floor antigens are reportedly liable for the homotypic adhesion of most cancers cells, and the most cancers cell membrane camouflage platform reveals splendid homotypic cancer-targeting capability [41]. CD47 on the platelet floor can sign “don’t eat me” to phagocytes, thus gaining immune evasion capability and conferring an extended half-life to the drug supply system [42]. To confirm the isotype focusing on capability and immune evasion capability of BPQDs-DOX@OPM, the mobile uptake of DiD-labeled BPQDs-DOX@OPM was evaluated utilizing Saos-2, 3T3, HBMEC, and RAW264.7 as controls. DID-(BPQDs-DOX@OPM) exhibited sturdy pink fluorescence after coincubation with Saos-2 cells for 4 h. No important fluorescence distribution was seen in 3T3, HBMEC, and RAW264.7 cells, indicating that BPQDs-DOX@OPM has OS cell isotype focusing on and immune evasion skills.
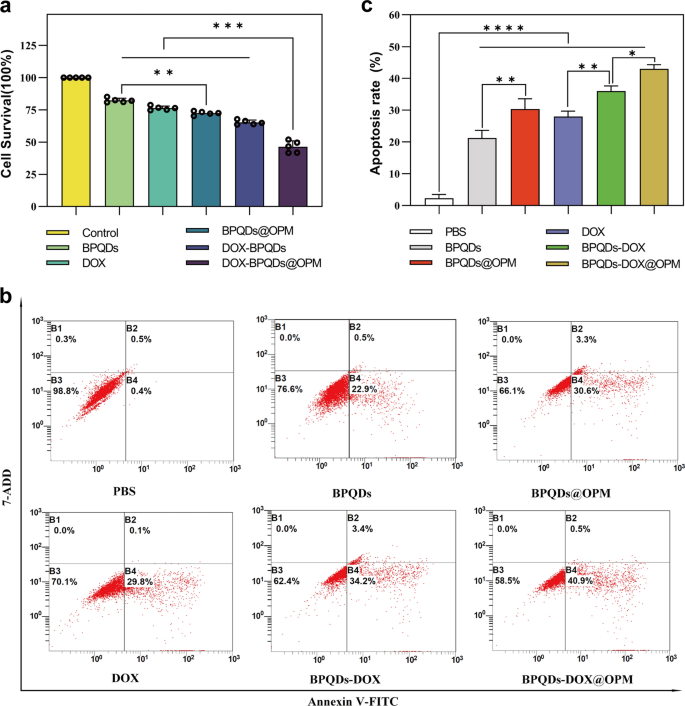
In vitro anti-tumor impact research of BPQDs-DOX@OPM
Impact of BPQDs-DOX@OPM on proliferative exercise of Saos-2 cells
Necrosis and apoptosis are the most typical types of cell dying. To analyze the in vitro anti-tumor impact of the BPQDs-DOX@OPM mixed drug supply system, we detected the proliferative exercise of Saos-2 by CCK-8 and the apoptosis fee by move cytometry. As proven in Fig. 5a, totally different teams have been set (i.e., Management, BPQDs, BPQDs@OPM, DOX, BPQDs-DOX, and BPQDs-DOX@OPM), and every group was irradiated with 808-nm NIR mild when including the drug. After treating Saos-2 cells for twenty-four h, the cell survival charges of the BPQDs and BPQDs@OPM have been 82.32% ± 1.54% and 72.48% ± 1.30%, respectively. The cell survival fee within the BPQDs@OPM group was decrease than that within the BPQDs group, indicating that the floor modification of the OPM might improve the soundness of BPQDs. BPQDs-DOX@OPM was considerably decrease, indicating that the mixed remedy had a stronger killing impact on OS.
BPQDs-DOX@OPM in vitro efficacy research. a Every remedy group handled with Saos-2 cells for twenty-four h, relative cell survival fee. b Apoptosis fee of Saos-2 cells handled with every remedy group for twenty-four h. c Statistical evaluation of apoptosis fee of Saos-2 cells. (*P < 0.05,**P < 0.01, ****P < 0.001)
Impact of BPQDs-DOX@OPM mixed drug supply system on apoptosis of Saos-2 cells
As proven in Fig. 5b, c, totally different teams (i.e., Management, BPQDs, BPQDs@OPM, DOX, BPQDs-DOX, and BPQDs-DOX@OPM) have been set as much as deal with Saos-2 cells for twenty-four h. Every group was irradiated with 808 nm NIR mild when the drug was added. In contrast with the apoptosis fee of the management group, these of the DOX, BPQDs@OPM, and BPQDs-DOX teams have been 28.00 ± 1.38%, 30.36 ± 2.64%, and 36 ± 1.35%, respectively. The BPQDs-DOX@OPM mixed remedy group had an apoptosis fee of 42.97 ± 1.13%, indicating that the mixed remedy had a extra highly effective killing impact on OS than single-agent remedy.
In vivo focusing on research of BPQDs-DOX@OPM
In vivo focusing on assay with in vivo optical imaging of small animals
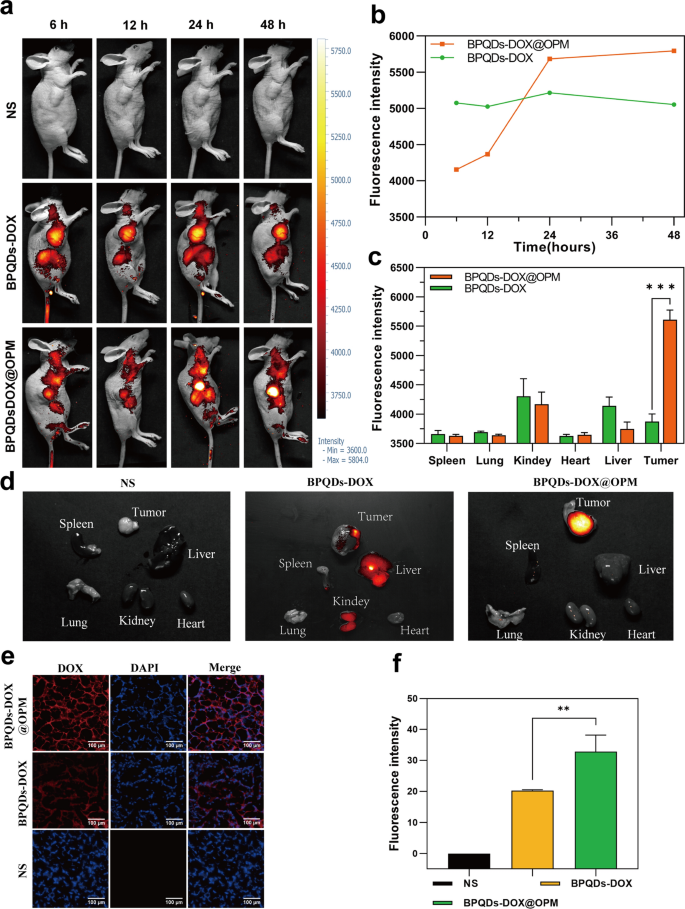
To additional verify the speculation of focusing on, the BPQDs-DOX@OPM mixture drug supply system was labeled with the fluorescent dye Cy5, and its in vivo biodistribution was detected by in vivo fluorescence imaging of small animals. Determine 6a, b reveals that BPQD-DOX partially accrued in tumor tissues at 6 and 12 h after drug injection in contrast with the NS group. That is because of the “passive focusing on” impact [43], and the aggregation of BPQDs-DOX in tumor tissues and main organs decreased at 48 h, in all probability owing to its smaller measurement and speedy metabolic clearance by the physique. In contrast with BPQDs-DOX, BPQDs-DOX@OPM confirmed important aggregation in tumor tissues 24 h after intravenous injection, and its aggregation in tumor tissues didn’t present a major lower after 48 h. As proven in Fig. 6c, d, tumors and main organs (i.e., coronary heart, liver, spleen, lungs, and kidney) have been collected 48 h after drug remedy. The fluorescence imaging outcomes confirmed that no apparent fluorescence distribution was noticed in all main organs of the BPQDs-DOX-treated group, and the fluorescence depth within the tumor tissues was low, indicating that BPQDs-DOX was quickly cleared by the organism at 48 h. The BPQDs-DOX@OPM-treated group confirmed an apparent fluorescence distribution within the tumor tissue however not in the primary organs. This indicated that BPQDs-DOX@OPM has good in vivo focusing on and lengthy circulation time.
BPQDs-DOX@OPM in vivo focusing on research. a, b Fluorescence depth and distribution in animals after tail vein injection of cy5-labeled BPQDs-DOX@OPM at 6, 12, 24, and 48 h. c, d Fluorescence distribution of tumor tissues and coronary heart, liver, spleen, lungs, and kidneys of animals 48 h after tail vein injection of cy5-labeled BPQDs-DOX@OPM. e Fluorescence distribution of DOX in animal tumor tissues 48 h after tail vein injection of cy5-labeled BPQDs-DOX@OPM. f Evaluation of DOX fluorescence depth in animal tumor tissues
DOX aggregation in tumor tissues
As proven in Fig. 6e and f, 48 h after OS mice have been handled with NS, BPQDs-DOX- and BPQDs-DOX@OPM, the tumor tissues have been taken to make frozen sections, and the sections have been restained with DAPI and noticed underneath a fluorescence microscope. The brilliant fluorescence of DOX was noticed within the BPQDs-DOX@OPM group, and the fluorescence depth was considerably stronger than that of the BPQDs-DOX group; this confirmed the superb focused drug supply efficiency of BPQDs-DOX@OPM.
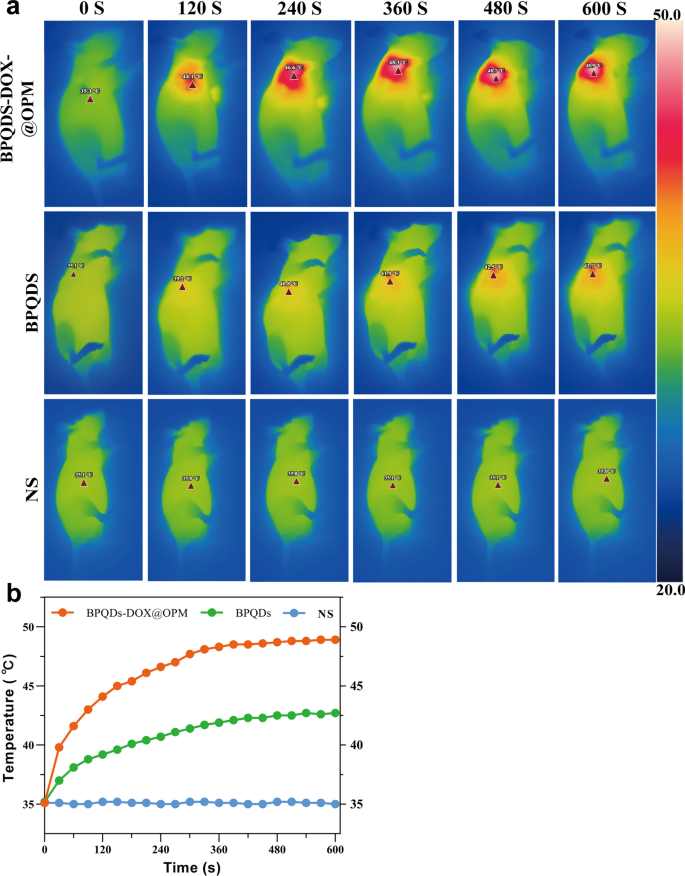
In vivo temperature rise curve of BPQDs-DOX@OPM
To additional examine the in vivo photothermal efficiency of the BPQDs-DOX@OPM co-loading system, three teams have been established: NS saline group, BPQDs group, and BPQDs-DOX@OPM group. Determine 7a, b depicts that every group was subjected to 808 nm near-infrared mild irradiation for 10 min. The NS group confirmed no important temperature change, whereas the BPQDs and BPQDs-DOX@OPM teams exhibited a speedy temperature improve throughout irradiation. After 10 min, the BPQDs and BPQDs-DOX@OPM teams reached 42.7 °C and 48.9 °C, respectively. The ultimate temperature of the BPQDs-DOX@OPM group was increased than that of the BPQDs group, surpassing the deadly temperature of tissue cells at 42 °C. This remark signifies that BPQDs-DOX@OPM demonstrated wonderful in vivo photothermal efficiency. The temperature elevation within the tumor space of the BPQDs-DOX@OPM group will be attributed to the mixed impact of efficient tumor focusing on and the excessive stability of BPQDs.
In vivo anti-tumor impact of BPQDs-DOX@OPM mixed drug supply system
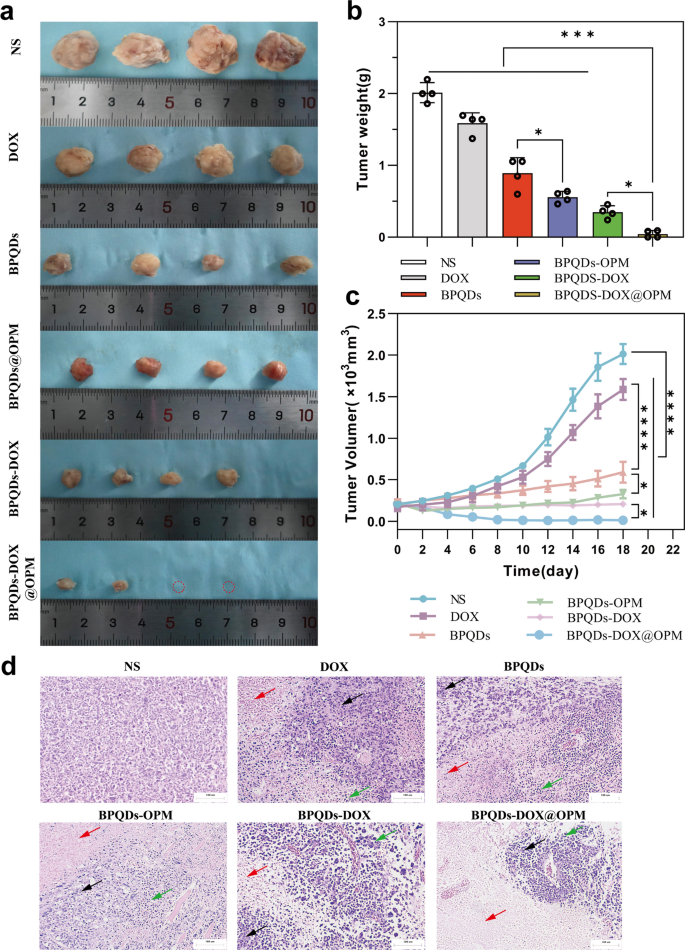
As proven in Fig. 8a–c, the in vivo therapeutic results of various remedy teams on OS have been clearly totally different. In contrast with the NS group, the BPQDs group and BPQDs@OPM group considerably diminished the speed of tumor quantity improve. That is because of the good therapeutic impact of PTT on native tumor development. The tumor quantity within the BPQDs@OPM group was considerably smaller (p < 0.05) than within the BPQDs group. This was as a result of the floor modification of OPM considerably improved the soundness and slowed down the oxidative degradation of BPQDs; this was per our preliminary assumption. The tumor development fee inhibition was extra evident within the BPQDs-DOX group than within the BPQDs and DOX teams, indicating that mixed chemotherapy with PTT has extra apparent benefits than a single-agent remedy for tumor remedy. Nonetheless, BPQDs-DOX could also be cleared by the immune system and renal excretory system in vivo owing to the immune clearance system and small measurement. As well as, BPQDs endure additional degradation upon publicity to oxygen and water, resulting in diminished efficacy. Our BPQDs-DOX@OPM mixed remedy group confirmed essentially the most pronounced tumor development inhibition on the finish of the remedy.
Anti-tumor results of BPQDs-DOX@OPM in vivo. a Tumor tissues after 18 days of intravenous injection of NS, BPQDs, DOX, BPQDs-@OPM, BPQDs-DOX, and BPQDs-DOX@OPM. b Measured tumor tissue weights after 18 days of remedy with NS, BPQDs, DOX, BPQDs-@OPM, BPQDs-DOX, and BPQDs-DOX@OPM. c Tumor quantity change curves throughout NS, BPQDs, DOX, BPQDs-@OPM, BPQDs-DOX, and BPQDs-DOX@OPM remedy. d H&E stained photos of tumor (measurement: 100 μm; Black arrows: aggregated tumor cell development; Pink arrows: tumor tissue necrosis; Inexperienced arrows: inflammatory cell infiltration.)
H&E staining of tumor tissue
Determine 8d illustrates the H&E staining of tumor tissues after the remedy. Within the NS group, tumor cells didn’t exhibit important necrosis and displayed various cell sizes, noticeable nuclear heterogeneity, and disordered association. In distinction, the remaining remedy teams exhibited tumor necrosis, intensified nuclear staining, and infiltration of inflammatory cells. Notably, the BPQDs-DOX@OPM group demonstrated in depth tumor cell necrosis, consolidation of nuclei with deepened staining, and sparse association of tumor cells with out nuclear schizogony. Furthermore, within the BPQDs-DOX@OPM group, there was widespread tumor cell necrosis, deepening of nuclear solidification staining, and infiltration of inflammatory cells within the residual tumor cells.
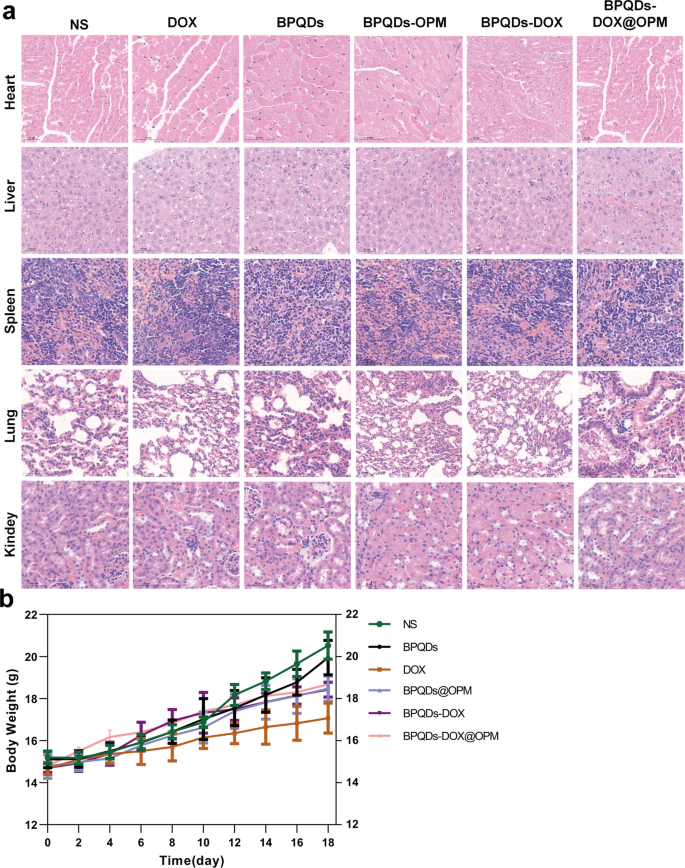
In vivo biosafety research of BPQDs-DOX@OPM mixed drug supply system
The poisonous results of nano-drug supply techniques on main organs and the entire system are thought-about a necessary course for assessing drug toxicity, and one among their main indicators is a change in physique weight [29]. As proven in Fig. 9a, the pattern of physique weight change in mice was recorded throughout remedy, and no important lower in physique weight was noticed within the BPQDs-DOX@OPM and BPQDs@OPM remedy teams. After the top of BPQDs-DOX@OPM in vivo remedy, the guts, liver, spleen, lungs, and kidneys of the sampled mice have been dissected. As proven in Fig. 9b, the H&E staining outcomes indicated that the BPQDs-DOX@ OPM group confirmed no important harm to all organs, indicating that the BPQDs-DOX@OPM mixed drug supply system had excessive biosafety.
In vivo biosafety evaluation of BPQDs-DOX@OPM. a H&E stained sections of every main organ of animals 18 days after intravenous injection of NS, BPQDs, DOX, BPQDs-@OPM, BPQDs-DOX, and BPQDs-DOX@OPM. b Physique weight adjustments of animals in every group throughout NS, BPQDs, DOX, BPQDs-@OPM, BPQDs-DOX, and BPQDs-DOX@OPM remedy. (measurement: 100 μm)