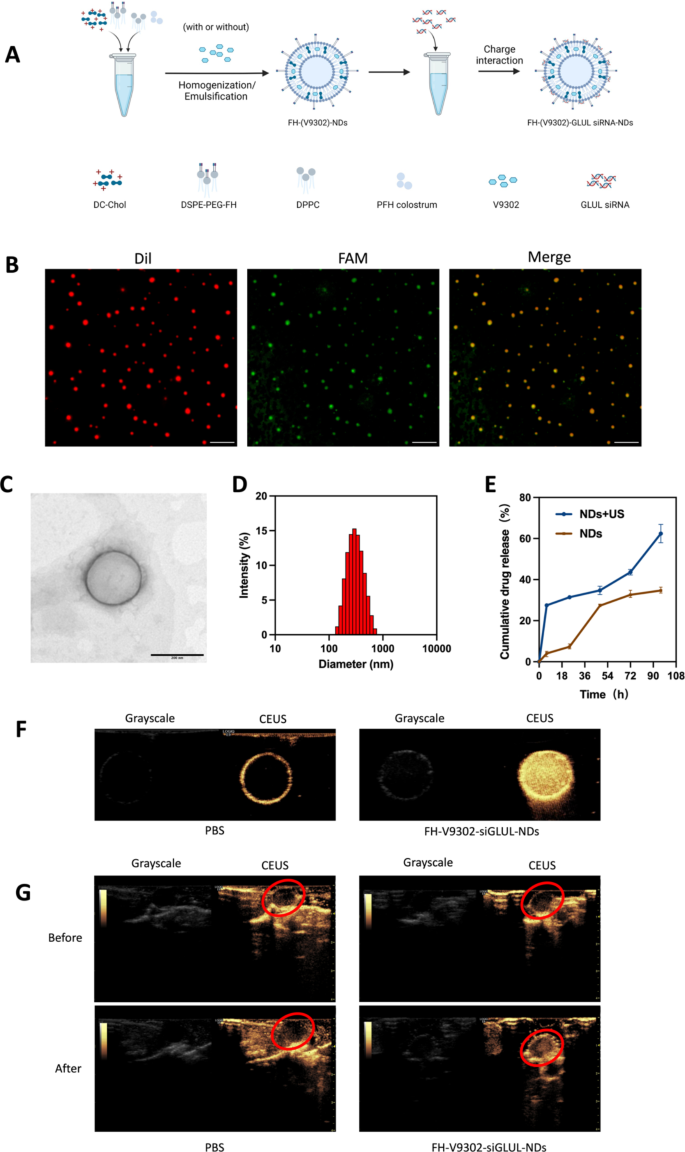
Preparation and characterization of the nanodroplets. (A) Preparation of the nanodroplets. (B) Fluorescence microscope diagram of FH-V9302-siGLUL-NDs. Scale bars: 10 μm. (C) Transmission electron microscopy (TEM) of FH-V9302-siGLUL-NDs at a quantity ratio of 1:20 ratio (siGLUL: nanodroplets). Scale bars: 200 nm. (D) FH-V9302-siGLUL-NDs dimension distribution. (E) Drug launch of FH-V9302-siGLUL-NDs with or with out ultrasound stimulation (0.5 W/cm2, 60 s, 1.0 MHz). (F) US imaging in vitro. (G) US imaging in vivo. The purple circle signifies the location of the tumor. All statistical knowledge are expressed as means ± SD (n = 3)
Characterization of nanodroplets
The DSPE-PEG-FH was assembled by the prior methodology [25, 26]. Primarily based on earlier research [27], V9302 and siGLUL-loaded and FH peptide-modified sonicated nanodroplets, named FH-V9302-siGLUL-NDs (Fig. 2A). Fluorescence microscopy photographs of FH-V9302-siGLUL-NDs are proven in Fig. 2B. FH-V9302-siFAM-NDs-Dil was ready by staining Dil and changing siGLUL with FAM siRNA (siFAM). Crimson dots represented nanodroplets, inexperienced dots represented siFAM, and yellow dots represented the profitable binding of FH-V9302-NDs-Dil and siFAM. The electron micrographs confirmed that the FH-V9302-siGLUL-NDs had been common spheres (Fig. 2C). The typical diameter of FH-V9302-siGLUL-NDs was 301.1 nm, and common polymer dispersion index (PDI) was 0.132 (Fig. 2D). Based on our examine, the EE and LE of FH-V9302-siGLUL-NBs had been 58.3% and 27.6%, respectively. The drug launch of FH-V9302-siGLUL-NDs was additionally studied. With out publicity to US stimulation, the ready FH-V9302-siGLUL-NDs exhibited sluggish drug launch, solely 34.7% of drug was launched inside 96 h. After publicity to US stimulation, V9302 was quickly launched, the cumulative launch exceeded 62.4% in 96 h (Fig. 2E).
The CEUI functionality of nanodroplets
The CEUI functionality of NDs might be visualized in actual time. To guage the potential of FH-V9302-siGLUL-NDs + US as a CEUI agent, US imaging was carried out in vivo and in vitro in grayscale and CEUS modes. Within the in vitro experiments (Fig. 2F), there was no important echogenic sign within the PBS group, in distinction, the echogenic sign of FH-V9302-siGLUL-NDs was considerably and considerably elevated. Within the in vivo experiments (Fig. 2G), the echogenic sign on the tumor web site was virtually unchanged after intravenous PBS injection. Nevertheless, the intravenous FH-V9302-siGLUL-NDs group confirmed a powerful enhance in echogenic sign on the tumor web site after injection. In conclusion, FH-V9302-siGLUL-NDs may enhance distinction imaging and obtain an built-in mode of prognosis and remedy.
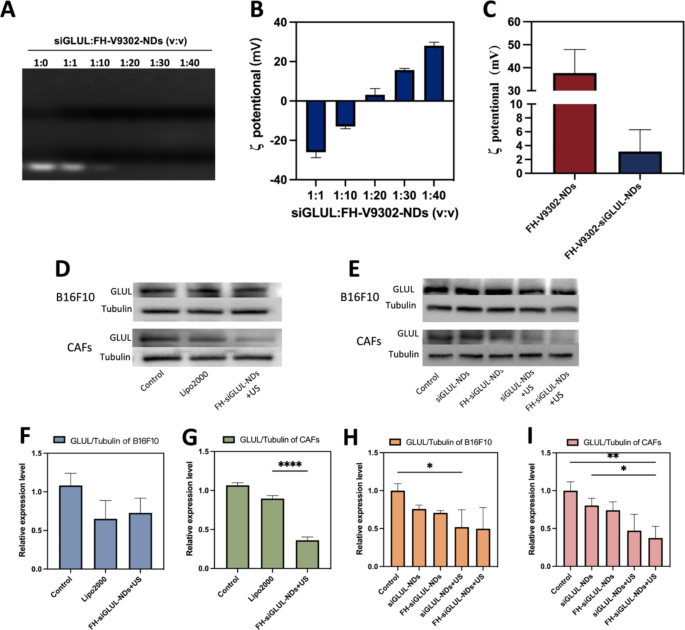
Characterization of FH-V9302-siGLUL-NDs and gene silencing effectivity. (A) Agarose gel electrophoresis retardation assay of FH-V9302-NDs with siGLUL in numerous quantity ratios. (B) ζ potential adjustments of siGLUL with FH-V9302-NDs at completely different quantity ratios (n = 3). (C) ζ potential of FH-V9302-NDs and FH-V9302-siGLUL-NDs (n = 3). (D) Western blot evaluation of B16F10 and CAFs cells with completely different remedy knockdown GLUL ranges. (E) Western blot evaluation of various nanodroplets loading siGLUL knockdown GLUL ranges. (F&G) Quantitative evaluation of GLUL expression in B16F10(F) and CAFs(G) cells below completely different therapies. (H&I) Quantitative evaluation of GLUL expression in B16F10 (H) and CAFs (I) cells handled with completely different nanodroplets loading siGLUL. *p < 0.05, **p < 0.01, ****p < 0.0001 (ANOVA take a look at). All statistical knowledge are expressed as means ± SD (n = 3)
Characterization of FH-V9302-siGLUL-NDs and gene silencing effectivity
The siGLUL was diluted to twenty µM and positively charged nanodroplets had been ready with constructive ldl cholesterol, and siGLUL was ready into nanodroplets at completely different quantity ratios by charging interplay. FH-V9302-siGLUL-NDs had been efficiently ready with completely different siGLUL: FH-V9302-NDs quantity ratios (1:0, 1:1, 1:10, 1:20, 1:30 and 1:40), and Fig. 3A confirmed that FH-V9302-NDs may absolutely load siGLUL when the ratio was lower than 1:20. The consequence confirmed the ζ potential of FH-V9302-siGLUL-NDs will increase with the lower of small disturbances (Fig. 3B). As the quantity ratio elevated from 1:10 to 1:20, the ζ potential modified from destructive to constructive, and siGLUL was absolutely loaded by constructive FH-V9302-NDs at a ratio of 1:20. Subsequently, a quantity ratio of 1:20 ratio (siGLUL: nanodroplets) was the optimum ratio for the preparation of nanodroplets. The ζ potential of FH-V9302-siGLUL-NDs was + 3.15 ± 3.16 mV (Fig. 3C). The ζ potential of FH-V9302-NDs with out siGLUL was + 37.67 ± 10.23 mV (Fig. 3C). Balancing the ζ-potential and siGLUL loading effectivity, a ratio of 1:20 was chosen to synthesize the FH-V9302-siGLUL-NDs.
Above outcomes instructed that the nanodroplets had the potential for gene silencing, which was additional validated in subsequent experiments. The gene silencing effectivity of nanodroplets was investigated. CAFs and B16F10 cells transfected with siGLUL by LipofectamineTM 2000 (Lipo2000-siGLUL) for six h had been used as constructive management. Preparations containing siGLUL had been incubated with cells for 15 min, then with or with out the appliance of ultrasound stimulation (0.5 W/cm2, 60 s, 1.0 MHz). After 6 h of incubation, the medium was changed with full medium and incubation continued for 48 h. Western blot evaluation was carried out with otherwise handled B16F10 and CAFs cells (Fig. 3D). The outcomes of their quantitative evaluation confirmed that for the B16F10 cells group (Fig. 3F), Lipo2000 had a greater silencing effectivity and the FH-siGLUL-NDs + US remedy was second solely to Lipo2000. For the CAFs cells group, though Lipo2000 had a superb gene silencing effectivity (Fig. 3G), the FH-siGLUL-NDs + US remedy had a good higher gene silencing effectivity, reaching 64%. This may very well be associated to the higher mobile uptake of FH-siGLUL-NDs by CAFs. To additional decide the effectivity of knockdown of GLUL, 5 teams of nanodroplets containing siGLUL had been designed to analyze the impact of gene silencing on B16F10 and CAFs (Fig. 3E). The Western blot outcomes had been additionally quantified, and completely different remedy teams may result in completely different decreases in GLUL ranges on B16F10 (Fig. 3H) and CAFs (Fig. 3I). The concentrating on potential of FH-NDs and mixed with ultrasound stimulation tremendously inhibited the expression of GLUL in CAFs cells. As a result of CAFs cells had good mobile uptake effectivity for FH-NDs. Ultrasound stimulation tremendously inhibited the expression of GLUL in B16F10 cells. In contrast with the PBS group, the GLUL relative expression ranges within the FH-siGLUL-NDs + US teams of B16F10 and CAFs had been 0.50 ± 0.28 and 0.37 ± 0.15, respectively. Nevertheless, within the absence of ultrasound stimulation, neither siGLUL-NDs nor FH-siGLUL-NDs considerably inhibited GLUL in B16F10 and CAFs cells. The above outcomes instructed that ultrasound stimulation may considerably enhance the silencing impact of GLUL in B16F10 and CAFs cells. The concentrating on potential may solely enhance the silencing impact of GLUL in CAFs cells, whereas it had no important impact on the silencing impact of GLUL in B16F10 cells.
Western blot evaluation was carried out with otherwise handled B16F10 cells (Fig. S1A). Quantification of ASCT2 (Fig. S1B) by western blot confirmed that within the V9302-siGLUL-NDs + US and FH-V9302-siGLUL-NDs + US group, the relative expression of ASCT2 in B16F10 cells was considerably inhibited, with relative expressions of 0.39 ± 0.25 and 0.31 ± 0.10, respectively. The relative expression of ASCT2 in FH-V9302-NDs group with relative expressions of 0.89 ± 0.13. The above outcomes can show that nanodroplets containing V9302 can inhibit the expression of ASCT2, whereas ultrasound stimulation can tremendously inhibit the expression of ASCT2. The weaker inhibitory impact of the FH-V9302-NDs group on the expression of ASCT2 than the free V9302 group could also be as a result of sluggish launch impact of the nanodroplets.
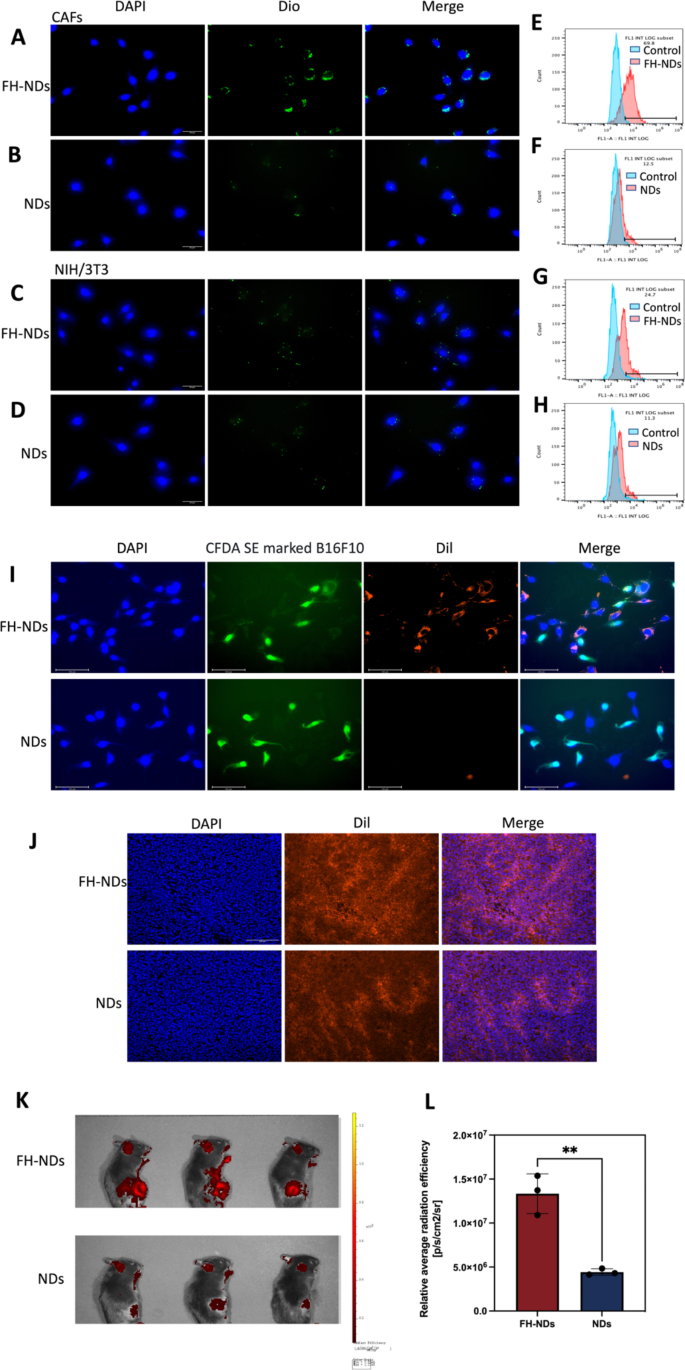
Focused potential of FH-NDs. (A&C) Fluorescence microscope diagram of the focused potential of FH-NDs. Scale bars: 30 μm. (B&D) Fluorescence microscope diagram of the focused potential of NDs. Scale bars: 30 μm. (E-H) Stream cytometry measured the focused potential of NDs and FH-NDs in CAFs and NIH/3T3 cells after incubation for one hour. (I) Fluorescence micrograph of the concentrating on potential of nanodroplets below co-culture situations of CAFs with B16F10. Scale bars: 100 μm. (J) Focusing on potential and accumulation of FH-NDs and NDs in tumor 4 h after injection of Dil-labelled nanodroplets. Scale bar: 200 μm. (Ok) Focusing on potential of FH-NDs and NDs in mice 4 h after injection of Dil-labelled nanodroplets. (L) Quantitative evaluation of concentrating on potential of FH-NDs and NDs in mice. **p < 0.01(t-test). All statistical knowledge are expressed as means ± SD (n = 3)
Focused potential of FH-NDs
To confirm the concentrating on potential of FH-NDs, Dio was chosen as a fluorescent probe. The concentrating on potential of FH-NDs was confirmed by fluorescence microscopy imaging and fluorescence-activated cell sorting. In fluorescence mode, nanodroplets labeled with Dio was inexperienced dot and nuclei stained with DAPI had been blue. As illustrated in Fig. 4A and B, in CAFs teams, many FH-NDs aggregated on CAFs, whereas fewer NDs aggregated on CAFs. Within the NIH/3T3 group, a small variety of FH-NDs aggregated on NIH/3T3 and fewer NDs aggregated on NIH/3T3 (Fig. 4C and D). Fluorescence-activated cell sorting additionally confirmed, in CAFs teams, the focused binding price of FH-NDs was considerably greater than NDs, 69.37 ± 1.79% vs. 12.30 ± 0.44%, respectively (****p < 0.0001) (Fig. 4E and F). In distinction, for the NIH/3T3 teams, the binding price of focused FH-NDs was considerably greater than untargeted NDs, 21.20 ± 4.51% and 11.3 ± 0.86%, respectively (*p < 0.05) (Fig. 4G and H). We studied the mobile uptake of focused and non-targeted nanodrops utilizing the in vitro co-culture system. B16F10 was labelled with CFDA SE exhibiting inexperienced fluorescence after which co-cultured with CAFs. The co-cultured cells had been incubated with Dil-labelled FH-NDs and NDs for one hour respectively. The mobile uptake of the nanodroplets was noticed by fluorescence microscopy images (Fig. 4I). Within the focused FH-NDs group, nanodroplets had been considerably aggregated in CAFs cells and barely in CFDA SE labelled B16F10 cells. Within the untargeted NDs group, there was no important aggregation of nanodroplets in both CAFs or B16F10 cells. These outcomes counsel that FH-NDs have a big concentrating on impact on CAFs cells. The FH peptide has been proven to have a wonderful excessive binding affinity for tenascin C, which is extremely expressed in CAFs [28]. Moreover, the concentrating on potential is correlated with tenascin C expression, which is low in NIH/3T3 cells and excessive in CAFs [29]. The consequence additional instructed important perform of FH peptide in concentrating on binding to CAFs.
To additional consider the in vivo concentrating on of FH-NDs, tumors transplanted to C57 mice had been used as a mannequin. It was noticed by fluorescence microscopy {that a} important intracellular purple fluorescence was noticed in frozen sections of tumors within the FH-NDs group, whereas much less was noticed in NDs group (Fig. 4J). As proven in Fig. 4Ok, the fluorescence sign of the FH-NDs group was extra centered on the tumor than that of the NDs group, indicating the nice concentrating on of FH-NDs. The fluorescence sign was quantified (Fig. 4L). In abstract, FH-NDs have good lively tumor concentrating on potential. The FH brief peptide considerably elevated the binding of focused FH-NDs to tumors. The concentrating on potential of FH-NDs correlated with CAFs in tumors. In contrast with NDs, FH-NDs may considerably accumulate in tumors in vivo, indicating a big concentrating on potential, deep penetration and good retention impact. In our examine, FH-NDs had been actively focused, which considerably enhanced the buildup in tumor animal fashions.
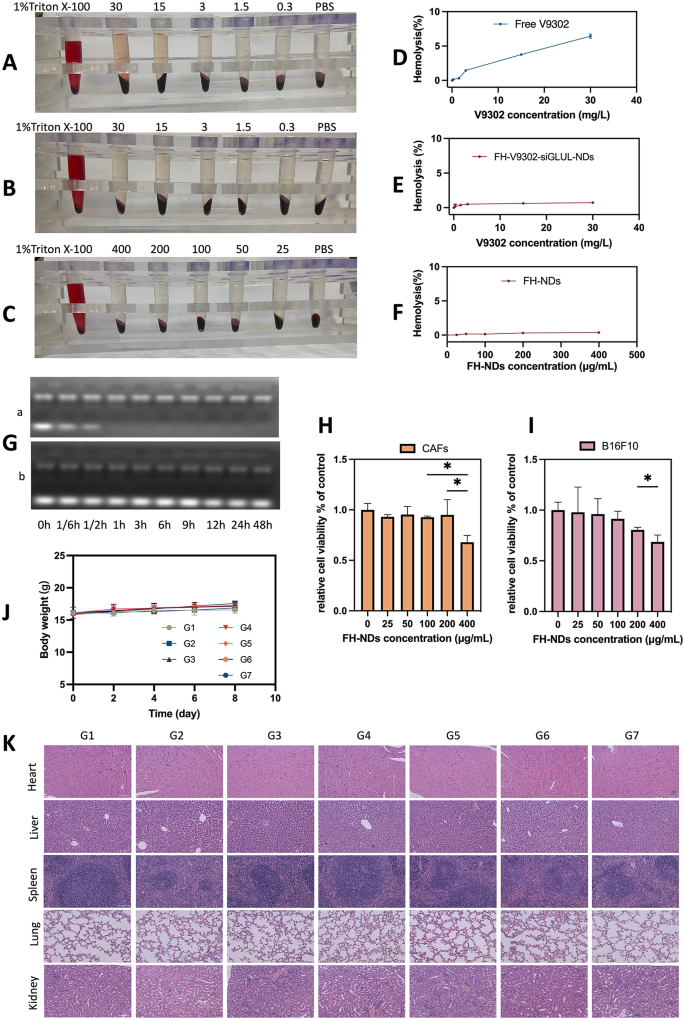
Security analysis. (A) Hemolytic conduct of Free V9302. (B) Hemolytic conduct of FH-V9302-siGLUL-NDs. (C) Hemolytic conduct of FH-NDs. (D) Statistics of hemolysis of Free V9302. (E) Statistics of hemolysis of FH-V9302-siGLUL-NDs. (F) Statistics of hemolysis of FH-NDs. (G) Agarose gel electrophoresis retardation assay of serum stability of bare siGLUL (a) and FH-V9302-siGLUL-NDs (b) in 50% fetal bovine serum. (H) Cytotoxicity of FH-NDs at completely different focus in CAFs. (I) Cytotoxicity of FH-NDs at completely different focus in B16F10. (J) Physique weight adjustments of mice between therapies (n = 5). (Ok) H&E staining of main organs after intravenous injection of FH-V9302-siGLUL-NDs. Scale bar: 100 μm. *p < 0.05 (ANOVA take a look at). All statistical knowledge are expressed as means ± SD (n = 3)
Security analysis
To substantiate the biosafety of FH-NDs, the next experiments had been carried out. The biosafety of the nanodroplets was demonstrated by hemolysis checks. The outcomes of blood erythrocyte lysis assays totally free V9302, FH-V9302-siGLUL-NDs, and clean FH-NDs are proven in Fig. 5A, B and C, respectively. The ends in Fig. 5D present that 30 mg/L of free V9302 produced a median hemolysis price of 6.43% in comparison with 0.73% induced by the identical focus of V9302 in FH-V9302-siGLUL-NDs (Fig. 5E). In distinction, the clean FH-NDs had the bottom hemolysis price which was 0.38% and had the very best blood compatibility (Fig. 5F). In comparison with FH-NDs, hemolysis price elevated after V9302 and siGLUL had been encapsulated, nevertheless it was nonetheless a lot decrease than free V9302. As well as, FH-V9302-siGLUL-NBs exhibited good serum stability. The free siGLUL band was considerably weakened inside 10 min after incubation, and the band was utterly absent by 1 h (Fig. 5G). This means that the free siGLUL was uncovered to excessive focus of FBS and was shortly and utterly degraded by the enzyme. Observing the FH-V9302-siGLUL-NDs below the identical FBS focus incubation, siGLUL band depth was considerably weakened after incubation, brilliant siGLUL bands had been nonetheless clearly noticed till 48 h. The outcomes indicated that constructive FH-NDs had a protecting impact on siGLUL. The cytotoxicity of clean FH-NDs was assessed by incubating CAFs (Fig. 5H) and B16F10 cells (Fig. 5I) with FH-NDs at completely different concentrations utilizing the CCK8 assay. The outcomes confirmed that important cytotoxicity occurred when the focus of clean FH-NDs reached 400µg/mL. The cell survival charges of the clean FH-NDs concentrations used on this examine had been all above 95%, indicating that FH-NDs utilized in our examine had been sufficiently secure.
To check the potential in vivo toxicity of nanodroplets, feminine C57 mouse fashions with tumors had been handled otherwise. No important adjustments in physique weight of the mice had been noticed in the course of the remedy interval (Fig. 5J). After remedy, histological examination of important organs confirmed no important tissue harm (Fig. 5Ok). General, nanodroplets exhibited an appropriate biosafety profile.
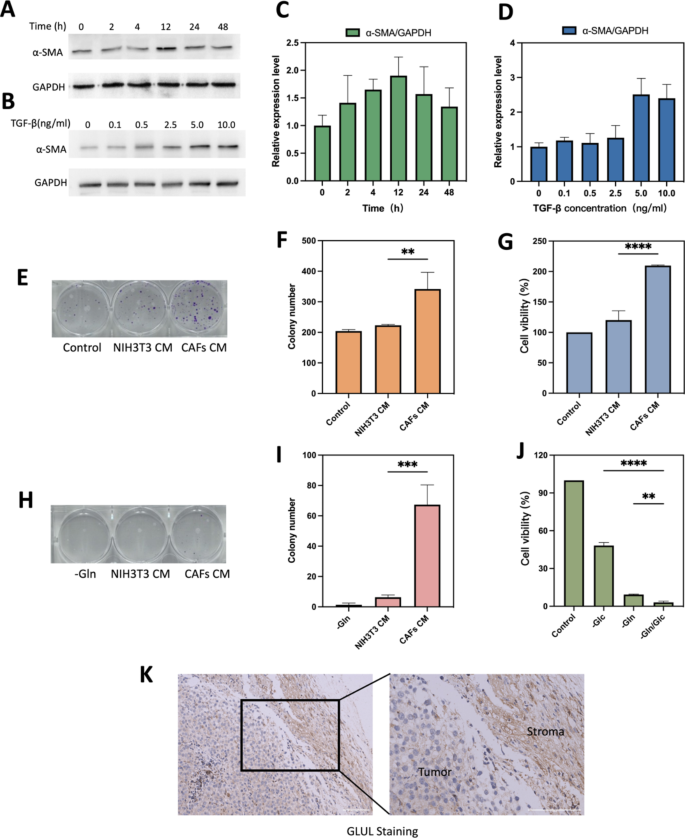
TGF-β1 induced CAFs and glutamine metabolism between CAFs and B16F10 cells. (A) Optimum time for induction of CAFs formation. (B) Optimum focus of TGF-β for induction of CAFs formation. (C) Quantification of various remedy occasions of α-SMA/GAPDH. (D) Quantification of various remedy focus of TGF-β of α-SMA/GAPDH. (E) Consultant photographs and (F)histograms of the impact of regular medium, NIH/3T3 CM and CAFs CM on the colony forming potential of B16F10 after 10 days of incubation. (G) Cell viability of various CM on B16F10 cell progress after 24 h of incubation. (H&I) B16F10, NIH/3T3 and CAFs had been cultured in Gln-deficient medium for six h. CAFs CM and NIH/3T3 CM had been taken to interchange the glutamate-free medium of B16F10 and the colony-forming potential was verified after 10 days. B16F10 cultured in glutamate-free medium was used as a management experiment. Consultant photographs(H) and histograms(I) of the impact of various CM on colony-forming capability of B16F10. (J)Cell viability of B16F10 cells with completely different situation medium. (Ok) IHC stained photographs evaluating GLUL protein expression between stromal and tumor compartments. Scale bars: 100 μm. CAFs CM is conditioned medium derived from CAFs, NIH/3T3 CM is conditioned medium derived from NIH/3T3. -Gln is the glutamine deprivation situation, -Glc is the glucose deprivation situation, and -Gln/Glc is the simultaneous glutamine and glucose deprivation situation. **p < 0.01, ***p < 0.001, ****p < 0.0001 (ANOVA take a look at). All statistical knowledge are expressed as means ± SD (n = 3)
NIH/3T3 efficiently induced into activated CAFs cells and glutamine metabolism between CAFs and most cancers cell
Mouse embryonic fibroblasts NIH/3T3 may very well be reworked into activated CAFs below sure situations [30]. Markers for CAFs are often easy muscle actin (α-SMA), fibroblast activating protein (FAP), platelet-derived progress issue receptor (PDGFR) α/β, fibroblast progress issue receptor (FGFR) and tenascin C (TNC) [31]. As the commonest used marker to label CAFs cells [32], α-SMA was chosen to confirm the profitable activation into CAFs in our examine. As proven in Fig. 6A and B, expression stage of α-SMA in TGF-β-stimulated serum-starved handled NIH/3T3 cells was considerably up-regulated at 12 h (Fig. 6C) or 5 ng/mL (Fig. 6D). Subsequently, serum hunger remedy of NIH/3T3 cells at 5 ng/mL of TGF-β1 for 12 h was used because the experimental situation for mimicking the tumor microenvironment.
The outcomes of plate clone formation experiments confirmed that the variety of clones shaped within the CAFs CM handled group was considerably greater than that within the NIH/3T3 CM handled group (Fig. 6E). CAFs CM may speed up the proliferation of B16F10 and enhanced their proliferation potential. The distinction was statistically important (P < 0.01) (Fig. 6F). As proven in Fig. 6G, CCK8 assay offered related outcomes to cloning experiments, cell viability within the CAFs CM group was stronger than within the NIH/3T3 CM group (209.8 ± 0.95% vs. 120.1 ± 15.38%; p < 0.0001). All of the above outcomes demonstrated that serum-starved handled NIH/3T3 cells may very well be transformed to practical CAFs below TGF-β1-stimulated situations. The outcomes of dietary deprivation experiments confirmed that the cell survival price of B16F10 was decrease below glutamine deprivation situations than below glucose deprivation situations, 9.34 ± 0.34% and 48.24 ± 2.39%, respectively (Fig. 6J). The outcomes and former analysis all instructed that B16F10 depends extra on glutamine metabolism than glycolytic metabolism [33]. And the outcomes of colony genesis experiments confirmed that CAFs CM rescued most cancers cells below glutamine-deficient situations, whereas NIH/3T3 CM barely rescued, 67.33 ± 13.0 vs. 6.33 ± 1.54, respectively (Fig. 6H and I). Could also be resulting from that CAFs may upregulate its glutamine synthesis and secrete glutamine into TME [30, 34]. Tumor metabolic reprogramming ends in altered glutamine metabolism between tumor and CAFs [35]. GLUL staining in mouse melanoma stable tumor tissues confirmed that GLUL expression was greater in fibrous stromal parts in comparison with tumor cells (Fig. 6Ok). The upper expression of GLUL in CAFs additionally implied that CAFs may play a task in synthesizing glutamine (Gln) to provide tumor cells and preserve the expansion of most cancers cells below nutrient-deficient TME [13].
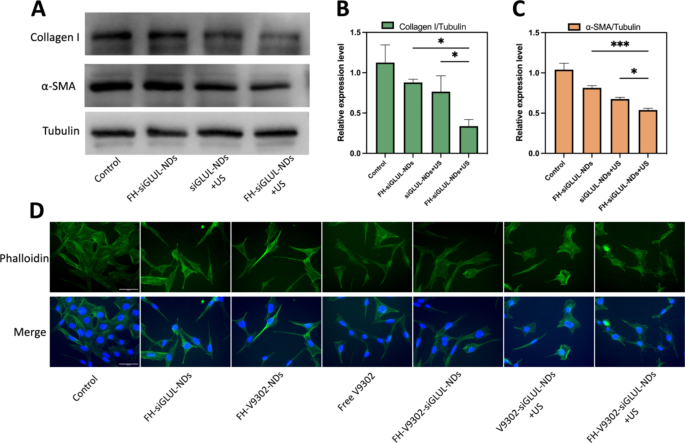
Inhibition of ECM and CAFs morphology after varied therapies (A) Western blot evaluation of collagen I and α-SMA after 48 h of CAFs handled with completely different parts containing siGLUL. (B) Quantitative evaluation of collagen I expression in CAFs. (C) Quantitative evaluation of α-SMA expression in CAFs. (D) Fluorescence microscope diagram of CAFs morphology 24 h after varied therapies. Scale bar: 50 μm. *p < 0.05, ***p < 0.001 (ANOVA take a look at). All statistical knowledge are expressed as means ± SD (n = 3)
Inhibition of ECM and CAFs morphology statement
It has been proven that blocking glutamine metabolism might severely disrupt your complete tumor metabolism, with a serious affect on TME [28]. CAFs may enhance the secretion of ECM, corresponding to collagen I. And a big and dense ECM may act as a bodily defend for the tumor, stopping drug penetration or infiltration into the tumor, resulting in poor efficacy [20]. On this examine, we discovered that the cytotoxicity of the nanodroplets containing V9302 on CAFs was lower than that of B16F10 cells, and the IC50 values of free V9302 on B16F10 and CAFs cells had been 33.52 µM and 69.26 µM, respectively. This indicated that V9302 didn’t kill CAFs immediately, however change it from an lively to a quiescent state, thus lowering the secretion of ECM [36]. Preparations containing siGLUL had been incubated with cells for 15 min, then with or with out the appliance of ultrasound stimulation (0.5 W/cm2, 60 s, 1.0 MHz). The cells had been incubated for 48 h. The inhibitory results of siGLUL-containing nanodroplets on α-SMA and collagen I had been verified by western blot (Fig. 7A). Quantification of collagen I (Fig. 7B) and α-SMA (Fig. 7C) by western blot confirmed that within the FH-siGLUL-NDs + US group, the relative expression of collagen I and α-SMA in CAFs cells was considerably inhibited, with relative expressions of 0.34 and 0.54, respectively. The above outcomes confirmed that siGLUL-containing nanodroplets may inhibit the formation of ECM, whereas ultrasound stimulation may promote the entry of siGLUL into cells and thus may considerably inhibit the formation of ECM. The flexibility of the focused group to inhibit ECM formation was greater than that of the non-targeted group, indicating that the focused mixed with ultrasound remedy group had a stronger potential to get rid of ECM.
Based on fluorescence microscopy imaging (Fig. 7D), simultaneous mixed glutamine metabolic blockade and ultrasound stimulation resulted in important adjustments in morphology of CAFs cells. The group containing V9302 modified CAFs from lively to quiescent state, and CAFs morphology turn into lengthy and lean. Mixed siGLUL and ultrasound stimulation modified the cell morphology irregular and chaotic. These outcomes demonstrated that ultrasound-responsive launch of V9302 and siGLUL may regulate cell dynamics by reorganizing the morphology and will inhibit ECM deposition.
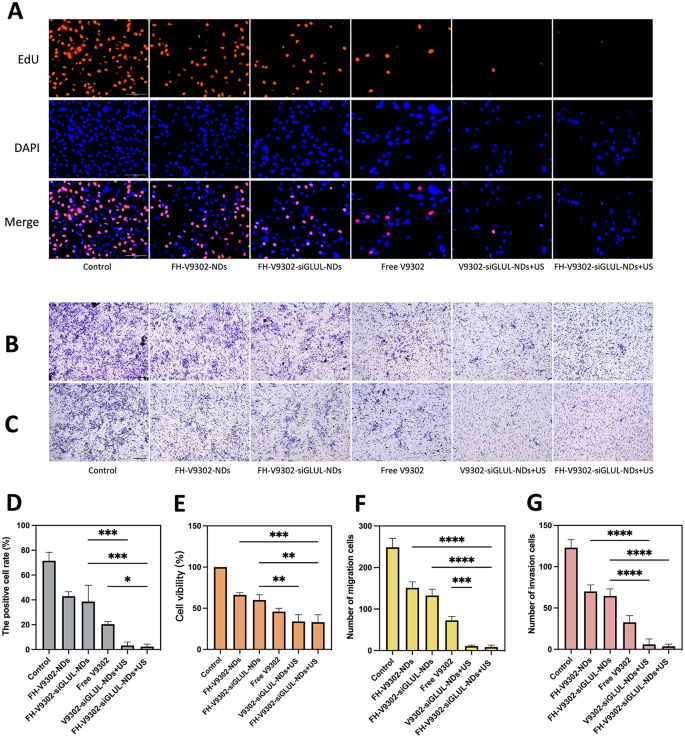
Anti-tumor potential in vitro. (A) Fluorescence photographs of EdU-positive cells after 24 h of varied therapies. Scale bar: 100 μm. (B&C) Handled B16F10 cells had been seeded on the higher chamber with or with out matrigel. After 24 h invaded cells had been detected and pictures captured and analyzed as described in Supplies and Strategies. Picture of migration (B) and invasion (C) cells examined by Transwell assay. Scale bar: 200 μm. (D) Quantification of the proportion of EdU-positive cells. (E) Cell viability of B16F10 cells after 24 h of varied therapies. (F&G) Quantification of the migrating cells (F) and invading cells(G). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001(ANOVA take a look at). All statistical knowledge are expressed as means ± SD (n = 3)
Antitumor efficacy in vitro
Blocking glutamine metabolism is a promising antitumor method [37]. The IC50 values of free V9302 on B16F10 cells had been 33.52 µM. At V9302 focus of 35 µM, the efficacy of the mix remedy on the proliferation of B16F10 cells was confirmed by the EdU assay (Fig. 8A). As proven in Fig. 8D, the proportion of EdU-positive cells within the FH-V9302-siGLUL-NDs + US group was the bottom, at 2.35 ± 2.06%, and the V9302-siGLUL-NDs + US group was 3.18 ± 2.86%. Most likely as a result of the FH-short peptide didn’t have a big concentrating on impact on B16F10 cells. The speed of EdU positivity was greater within the FH-V9302-siGLUL-NDs and FH-V9302-NDs teams than within the free V9302 group, most likely as a result of that V9302 was wrapped by nanodroplets and slowly launched with out ultrasound stimulation. The FH-V9302-siGLUL-NDs group inhibited proliferation to some extent than the FH-V9302-NDs group, most likely as a result of inhibition of GLUL [11]. Then, the cell viability was measured by the CCK8 methodology to measure the effectivity of various remedy (Fig. 8E). In contrast with different teams, the cell viability of FH-V9302-siGLUL-NDs + US group was the bottom at 33.11 ± 9.01%, adopted by V9302-siGLUL-NDs + US group at 33.94 ± 8.16% and FH-V9302-siGLUL-NDs at 59.86 ± 6.50%, indicating that the FH-short peptide had no impact on B16F10 and ultrasound stimulation promoted drug launch tremendously inhibiting cell viability. In conclusion, all teams containing V9302 clearly inhibited the proliferation and cell viability of B16F10 cells. All outcomes instructed that UTMD mixed with glutamine metabolic interruption had wonderful anti-tumor capability in vitro.
The migratory (Fig. 8B) and invasive (Fig. 8C) capability of the cells was assessed in vitro utilizing transwell assay. Determine 8 F confirmed the V9302-siGLUL-NDs + US group (11.00 ± 2.0) and the FH-V9302-siGLUL-NDs + US group (8.33 ± 4.50) had a low variety of migrating cells. Comparatively, the FH-V9302-NDs, FH-V9302-siGLUL-NDs and free V9302 teams had 151.0 ± 14.42, 132.7 ± 15.18 and 73.67 ± 9.60 migrating cells, respectively. As proven in Fig. 8G, the outcomes of the invasion assay confirmed related developments to the migration assay. These findings instructed that UTMD mixed with glutamine metabolism inhibition may successfully inhibit tumor migration and invasion potential. In conclusion, all teams containing V9302 may considerably inhibit the migration and invasion of B16F10. The presence or absence of FH-short peptide had little impact on the migration and invasion of B16F10 cells.
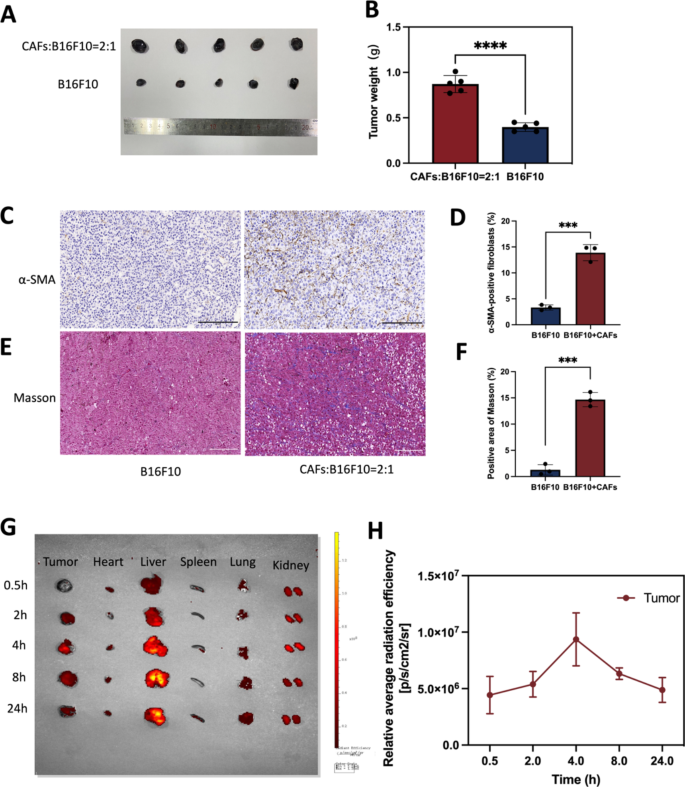
The institution of a tumor mannequin with co-implantation of B16F10 and CAFs cells and biodistribution evaluation of nanodroplets. (A) B16F10 blended with or with out CAFs cells had been injected intradermally into flanks of C57 mice, respectively. Images of tumor mannequin after 10 days. (B) Statistics of tumor weight. (C) IHC staining of α-SMA. Scale bar: 200 μm. (D) Quantitative evaluation of α-SMA utilizing Picture J software program. (E) Masson trichrome staining. Scale bar: 200 μm. (F) Quantitative evaluation of collagen deposition utilizing Picture J software program. (G) Biodistribution of nanodrugs within the physique 4 h after tail vein injection. (H) Quantitative evaluation of biodistribution of nanodrugs in tumor. ***p < 0.0001, ****p < 0.0001(t-test). All statistical knowledge are expressed as means ± SD (n = 3)
Biodistribution of nanodrugs in vivo
In vitro, it has been proven that TGF-β1 can induce the conversion of NIH/3T3 into CAFs, then mimicking TME in vivo was the query to be investigated on this examine. Based on current research [38,39,40], B16F10 and CAFs cells had been injected into mice at a 1:2 ratio on this experiment. Mouse fashions of co-inoculated melanoma was constructed with B16F10 blended with or with out CAFs cells, and 10 days later, tumors had been collected and ready for immunohistochemistry assess CAFs expression ranges. The outcomes confirmed that the tumor dimension of the blended transplanted tumor group was considerably bigger than that of the transplanted B16F10 alone (Fig. 9A). Statistics of tumor weight was confirmed in Fig. 9B(p < 0.0001). As proven within the Fig. 9D&F, co-cultured tumors contained roughly 13% CAFs in whole management cells. In contrast with the tumor mannequin inoculated with B16F10 alone, α-SMA (a CAF-labeled protein) expression was elevated within the tumor mannequin inoculated with B16F10 blended with CAFs (Fig. 9C). The expression of collagen secreted by CAFs was elevated in tumor fashions inoculated with B16F10 blended with CAFs in comparison with tumor fashions inoculated with B16F10 alone (Fig. 9E). The ultimate co-culture mannequin had a small proportion of CAFs and nearly all of tumor cells, most likely as a result of the expansion price of most cancers cells and CAFs cells was completely different. Tumor fashions co-cultured with B16F10 cells and CAFs cells is much like the actual melanoma in vivo greater than tumor fashions with B16F10 alone. We additionally verified the syngeneic mannequin by immunostaining for S100 expression. S100 calcium-binding protein B (S100-β) is a selected protein in melanoma. Fig. S2 exhibits apparent constructive price in melanoma tumor. As this examine used mouse melanoma and CAFs, future research may delve into the outcomes noticed in TME of human melanoma.
Inspired by the wonderful tumor concentrating on potential of FH-V9302-siGLUL-NDs, the biodistribution of nanodroplets in a mouse tumor mannequin was additional investigated. The very best peak distribution of Dil-labelled FH-V9302-siGLUL-NDs was reached within the tumor 4 h after intravenous injection. Even after 24 h, fluorescent alerts had been nonetheless current in tumor tissues, indicating the buildup of long-term retention of FH-V9302-siGLUL-NDs and deep penetration into the tumor (Fig. 9G). The fluorescence sign was quantified (Fig. 9H). Subsequently, in subsequent experiments, US irradiation was carried out on tumors 4 h after FH-V9302-siGLUL-NDs administration. As well as, the liver confirmed the very best fluorescence depth amongst regular organs and collected step by step over time, indicating that the liver captured the lipid nanodroplets. We guessed that the interplay between constructive nanodroplets and destructive cell membranes impact the clearance price [41, 42]. Though some research have reported longer half-life for cationic MBs [43], you will need to stability their interplay and retention.
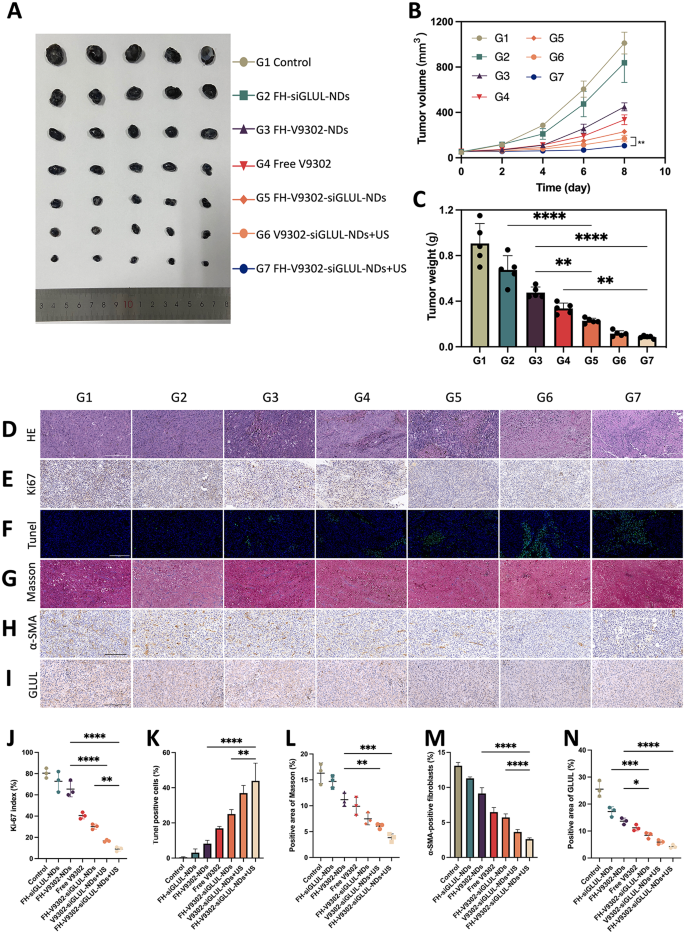
In vivo antitumor efficacy and reprogramming of TME. (A) Images of tumor shaped by B16F10 and CAFs after 4 therapies. (B) Modifications of tumor quantity in the course of the remedy (n = 5) **p < 0.001(t-test). (C) Weight of tumors shaped by B16F10 and CAFs after 4 therapies (n = 5). (D) H&E staining. Scale bar: 200 μm. (E) Ki67 staining. Scale bar: 200 μm. (F) Tunel staining. Scale bar: 200 μm. (G) Masson trichrome staining. Scale bar: 200 μm. (H) IHC staining of α-SMA. Scale bar: 200 μm. (I) IHC staining of GLUL. Scale bar: 200 μm. (J) Ki67 index evaluation (n = 3). (Ok) Quantitative evaluation of Tunel constructive cells (n = 3). (L) Evaluation of constructive space of Masson (n = 3). (M) Quantitative evaluation of α-SMA (n = 3). (N) Evaluation of constructive space of GLUL (n = 3). * p < 0.05, ** p < 0.01, and *** p < 0.001 (ANOVA take a look at). All statistical knowledge are expressed as means ± SD (n = 5). G1, Management; G2, FH-siGLUL-NDs; G3, FH-V9302-NDs; G4, Free V9302; G5, FH-V9302-siGLUL-NDs; G6, V9302-siGLUL-NDs + US; and G7, FH-V9302-siGLUL-NDs + US
In vivo antitumor efficacy
Feminine C57 mice carrying tumors had been handled otherwise to analyze the anti-tumor impact. As proven in Fig. 10A and B, in comparison with the management, the FH-siGLUL-NDs group exhibited modest tumor inhibition. The all handled teams containing V9302, in comparison with the FH-siGLUL-NDs group, exhibited important tumor suppression. We additionally discovered that the free V9302 group exhibited stronger tumor suppression than the FH-V9302-NDs group, which was primarily as a result of slow-release potential of the nanodroplets with out US stimulation. As anticipated, the FH-V9302-siGLUL-NDs + US-treated group confirmed the strongest tumor suppression, adopted by V9302-siGLUL-NDs + US with 9.34% and 12.78% of tumor weight of the management group, respectively (Fig. 10C). Ultrasound mixed with metabolic inhibition considerably inhibited tumor progress. In the meantime, selective abrogation of glutamine metabolism in CAFs in vivo has been proven to inhibit the expansion of ovarian tumors in mice [13]. In our experiments, important inhibition of tumor progress was noticed with V9302 remedy alone. Notably, the mix of V9302 plus siGLUL or V9302 plus siGLUL plus ultrasound stimulation resulted in higher inhibition of tumor progress, implying an extra impact of metabolic hunger remedy mixed with blockade of glutamine synthesis from CAFs. Our work offering a chance to focus on each the CAFs and most cancers cells to enhance remedy outcomes.
To confirm the anti-tumour impact, H&E (Fig. 10D), Ki-67(Fig. 10E) and Tunel (Fig. 10F) staining of tumour tissues was carried out. Determine 10D confirmed FH-V9302-siGLUL-NDs + US group had the biggest amount of tumour cell necrosis of all teams. Ki-67 additionally confirmed it had essentially the most pronounced inhibitory impact on tumor cell proliferation (Fig. 10J) and Tunel staining confirmed it had strongest pro-apoptotic impact (Fig. 10Ok). The above outcomes instructed that mixed ultrasound with reworking of glutamine metabolism may exhibit good antitumor results.
TME reworking
In an effort to reveal the mechanisms of the anti-tumour results of TME glutamine metabolic reprogramming, TME was evaluated. Tumor ECM expression was assessed by immunohistochemistry (IHC) of α-SMA and Masson trichrome staining for collagen [44]. As proven in Fig. 10G and H, in management group, a big quantity of collagen and α-SMA constructive fibroblasts was discovered settling within the intercellular stroma, whereas it was virtually absent within the FH-V9302-siGLUL-NDs + US group. As well as, GLUL in tumors was additionally measured by IHC (Fig. 10I). All nanodroplets containing V9302 can be utilized in considerably lowering ECM expression. As proven in Fig. 10L, V9302-siGLUL-NDs + US and FH-V9302-siGLUL-NDs + US downregulated expression of collagen to six.06 ± 0.50% and three.83 ± 0.74%, respectively. As proven in Fig. 10M, V9302-siGLUL-NDs + US and FH-V9302-siGLUL-NDs + US downregulated expression of α-SMA to three.64 ± 0.35% and a pair of.61 ± 0.19%, respectively. The stronger impact could also be resulting from the truth that ultrasound stimulated the immediate launch of V9302 and siGLUL and successfully eradicated ECM, thereby enhancing the nanodroplets infiltration within the tumor. As proven in Fig. 10N, V9302-siGLUL-NDs + US and FH-V9302-siGLUL-NDs + US downregulated expression of GLUL to five.93 ± 0.80% and 4.23 ± 0.66%, respectively. The stronger impact of FH-V9302-siGLUL-NDs + US could also be resulting from its higher concentrating on to tumor stroma. Subsequently, we concluded that metabolic inhibition of FH-V9302-siGLUL-NDs mixed with UTMD may down-regulate GLUL and successfully degrade ECM.