| Mar 17, 2023 |
|
(Nanowerk Information) They’re thought-about the “Holy Grail” of battery analysis: so-called “solid-state batteries”. They now not have a liquid core, as is the case with immediately’s batteries, however include a stable materials. This results in a number of benefits: Amongst different issues, these batteries are tougher to ignite and can be manufactured on a miniature scale.
|
|
Scientists on the Max Planck Institute for Polymer Analysis have now turned their consideration to the life cycle of such batteries and focused processes that scale back it. With their findings, extra sturdy solid-state batteries might be realized sooner or later.
|
|
Their findings have been revealed in (Nature Communications, “Understanding the evolution of lithium dendrites at Li6.25Al0.25La3Zr2O12 grain boundaries by way of operando microscopy methods”).
|
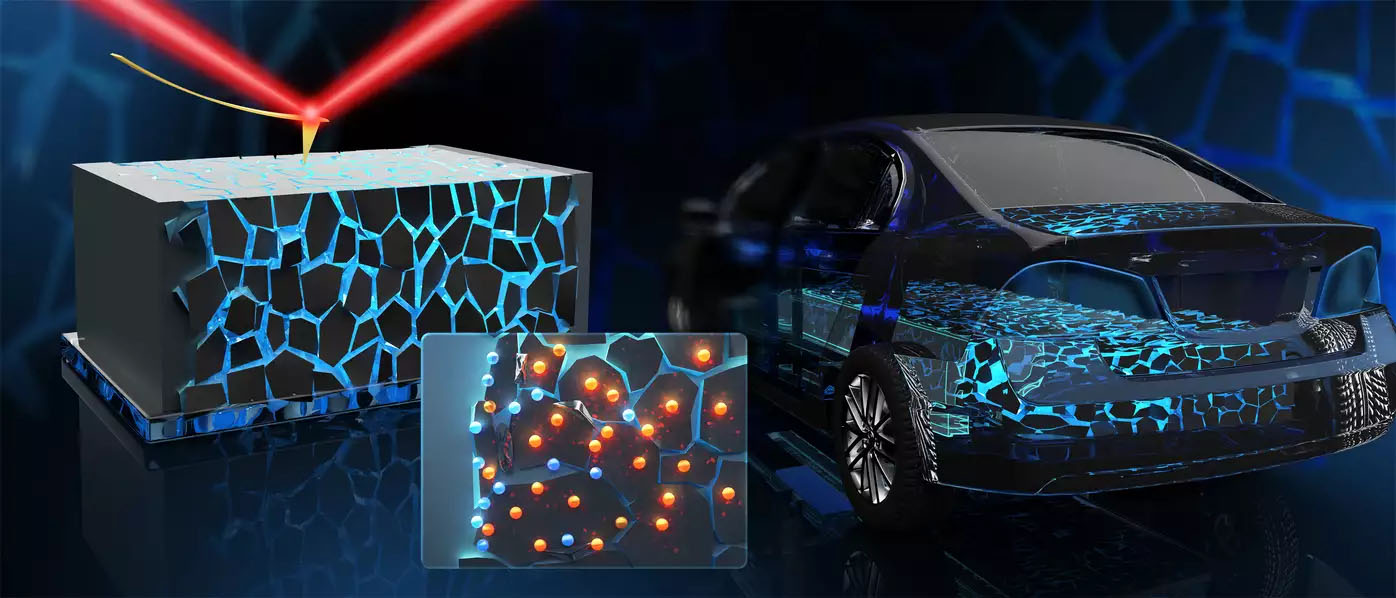 |
| Stable-state batteries might provide many benefits sooner or later, together with for the use in electrically powered vehicles. (Picture: Max Planck Institute for Polymer Analysis)
|
Whether or not in an e-car, cellphone, or cordless screwdriver, many units used every day now use rechargeable batteries. Nonetheless, the pattern additionally has its downsides. For instance, sure cell telephones had been banned from being carried on airplanes, or e-cars caught hearth. Fashionable business lithium ion batteries are delicate to mechanical stress.
Stable-state batteries might provide many benefits sooner or later, together with for the use in electrically powered vehicles
|
|
So-called “solid-state batteries” might present a treatment. These now not include a liquid core – the so-called electrolyte – however consist completely of stable materials, e.g. ceramics ionic conductor. Consequently, they’re mechanically sturdy, non-flammable, simple to miniaturize and insensitive to temperature fluctuations.
|
|
However solid-state batteries present their issues after a number of charging and discharging cycles: Whereas the constructive and unfavorable poles of the battery are nonetheless electrically separated from one another at the start, they’re ultimately electrically linked to one another by inside battery processes: “Lithium dendrites” slowly develop within the battery. These lithium dendrites develop step-by-step throughout every charging course of till the 2 poles are linked. The consequence: the battery is brief circuited and “dies”. Up to now, nonetheless, the precise bodily processes that happen on this course of usually are not but nicely understood.
|
|
A staff led by Rüdiger Berger from Hans-Jürgen Butt’s division has now tackled the issue and used a particular microscopy methodology to research the processes in additional element. They investigated the query of the place the lithium dendrites begin to develop. Is it like in a flowstone cave the place stalactites develop from the ceiling and stalagmites from the ground till they be part of within the center and kind a so-called “stalagnate”? There isn’t any prime and backside in a battery – however do dendrites develop from the unfavorable to the constructive pole or from the constructive to the unfavorable pole? Or do they develop equally from each poles? Or are there particular locations within the battery that result in nucleation after which dendritic progress from there?
|
|
Rüdiger Berger’s staff seemed specifically at so-called “grain boundaries” within the ceramic stable electrolyte. These boundaries are shaped in the course of the manufacturing of the stable layer: The atoms within the crystals of the ceramic are principally very recurrently organized. Nonetheless, attributable to small, random fluctuations in crystal progress, line-like constructions are shaped the place the atoms are organized irregularly – a so-called “grain boundary”.
|
|
These grain boundaries are seen with their microscopy methodology – “Kelvin Probe Drive Microscopy” – through which the floor is scanned with a pointy tip. Chao Zhu, a PhD pupil working with Rüdiger Berger says: “If the solid-state battery is charged, the Kelvin Probe Drive Microscopy sees that electrons accumulate alongside the grain boundaries – particularly close to the unfavorable pole.” The latter signifies that the grain boundary not solely adjustments the association of the atoms of the ceramics, but additionally their digital construction.
|
|
As a result of accumulation of electrons – i.e. unfavorable particles – positively charged lithium ions touring within the stable electrolyte will be lowered into metallic lithium. The consequence: lithium deposits and lithium dendrites kind. If the charging course of is repeated, the dendrite will proceed to develop till lastly the poles of the battery are linked. The formation of such preliminary levels for dendrite progress was solely noticed on the unfavorable pole – additionally noticed solely at this pole. No progress was noticed on the reverse constructive pole.
|
|
The scientists hope that with a exact understanding of the expansion processes, they can even be capable to develop efficient methods to stop or a minimum of restrict progress on the unfavorable pole, in order that sooner or later the safer lithium solid-state batteries can be utilized in broadband purposes.
|


