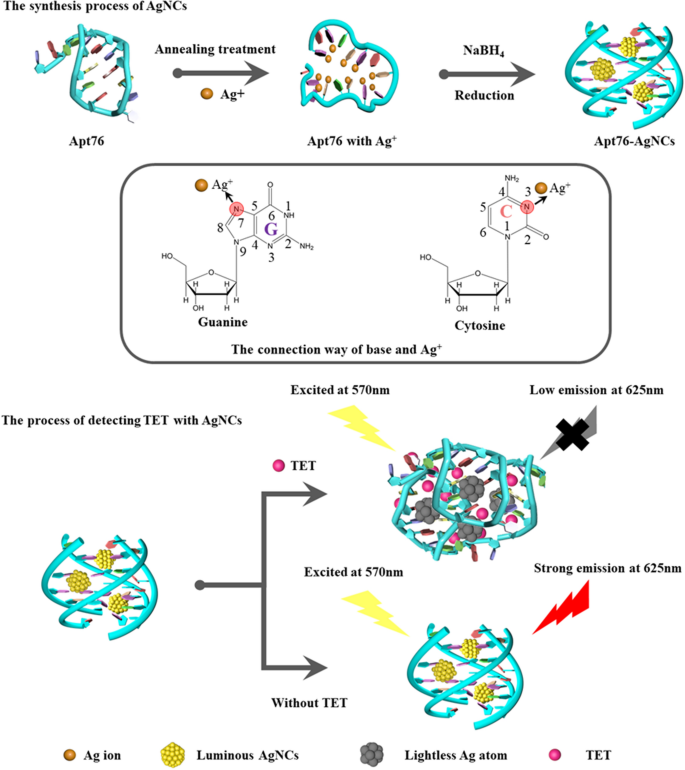
Precept of the label-free biosensor primarily based on AgNCs
The capability of Apt76 to provide AgNCs underpins the confirmed label-free aptamer biosensor. As proven in Scheme 1, the established aptamer biosensor is utilizing the power of Apt76 to fabricate AgNCs, target-induced Apt76 conformation transition, and optical properties of AgNCs various with the Apt76 conformation. The rationale why DNA may very well be used as a template for AgNCs is that silver ions can mix with the N3 place of the cytosine bases and the N7 and O6 positions of the guanine bases are additionally most well-liked binding websites for silver ions [28]. Furthermore, the DNA conformation is strongly influenced by sequence composition and impacts the expansion of AgNCs [29]. The content material of G and C bases in Apt76 is as excessive as 68.4%, which supplies a basis for the synthesis of AgNCs. As well as, it has been reported that Apt76 in buffer can type a G-quadruplex construction [30], which is an appropriate matrix for stopping AgNCs aggregation [21]. On this research, AgNCs synthesized with Apt76 as a template exhibited excessive fluorescence emission at 625 nm underneath stimulation at 570 nm. When goal molecules (TET) have been current, the form of the aptamer particular for TET modified from an antiparallel G-quadruplex to a hairpin construction. TET is quantified primarily based on the noticed lower in fluorescence emission at 625 nm produced by a change in Apt76 conformation upon particular binding to TET, which is adopted by a rise within the measurement of the encapsulated AgNCs.
Molecular docking outcomes of Apt76 and TET
Exploring the Apt76 recognition course of requires revealing the precise binding websites of Apt76 with TET. AutoDock Instruments was used to carry out a molecular docking investigation of Apt76 and TET. As seen in Fig. 1E, the 50 Apt76 conformations with various binding energies have been simulated and distributed as 33 clusters, and the conformation signed with pink column had the bottom binding power and the perfect potential of functioning as the actual conformation. The TET conformation comparable to the pink column is proven in Fig. 1C. Hydrogen bonding, form matching, fragrant ring stacking, electrostatic adsorption and van der Waals contacts are all used within the identification of nucleic acid aptamers and goal molecules, with hydrogen bonding being probably the most important [31]. The worldwide views depicted in Fig. 1D illustrate that Apt76 folds to type ‘pocket’ websites to encapsulate TET. As proven in Fig. 1F, Apt76 resembles a fancy s-shaped construction with the ligand-binding website within the despair of neck-loop stacking. Foremost contact happens at thymine T11, which types two hydrogen bonds with the 6β-OH group of tetracycline. The β-hydroxyketone moiety of TET is deeply buried in Apt76 and types three hydrogen bonds with T10, C51 and A50. As well as, T38 and G3 additionally shaped two hydrogen bonds with TET. The positions of hydrogen bonding on Apt76 and TET are marked in Fig. 1A, B, respectively. In response to the outcomes of the foregoing investigation, the amount of binding free power and the variety of hydrogen bonds demonstrated Apt76’s important binding capability to TET.
Optimization of Apt76-AgNCs synthesis circumstances
The fluorescent property of DNA-AgNCs is the supply of the biosensor sign, and the very best fluorescence depth corresponds to the perfect detection sensitivity, because of this, varied important variables for the creation of Apt76-AgNCs have to be optimized, together with the synthesis buffers with totally different pH values and Apt76/Ag+/NaBH4 molar ratios. Fluorescence depth and fluorescence quenching effectivity have been achieved as high quality indicators for AgNCs. First, the synthesis buffer for the synthesis of AgNCs was optimized. NH4Ac, PB and sodium citrate, that are generally used to organize AgNCs, have been chosen as synthesis buffers for comparability. Determine 2A exhibits the fluorescence depth at 625 nm of AgNCs produced with varied buffers underneath 570 nm excitation and fluorescence quenching effectivity within the presence of 10 µg/mL TET. It may be seen from Fig. 2A that underneath robust acidic and alkaline environments, it isn’t conducive to the synthesis of AgNCs. It is because N3 on cytosine extra simply binds to Ag+ close to impartial pH [32]. Apt76-AgNCs ready in all three buffers have the very best fluorescence emission depth at pH = 6.8, however solely in NH4Ac buffer have the very best fluorescence quenching effectivity when reacting with TET. This consequence is likely to be attributable to the truth that Na+ in PB and sodium citrate stabilize the G-quadruplex of Apt76 extra strongly than NH4+ in NH4Ac, and when reacting with TET, the secure construction is tougher to vary. In consequence, the lower in fluorescence depth is diminished [33]. Determine 2B depicts the affect of assorted DNA to Ag+ and NaBH4 ratios on the fluorescence depth of AgNCs. With a molar ratio of Apt76:Ag+:NaBH4 of 1:12:3, Apt76-AgNCs confirmed the utmost fluorescence depth in addition to the best lower in fluorescence sign when TET is current. The outcomes point out that underneath the identical molar ratio of Apt76 and Ag+, the decreasing agent focus is just not proportional to the fluorescence sign, as a result of an extra focus of decreasing agent causes an excessive amount of Ag+ to be diminished to Ag atoms, which transforms AgNCs into AgPCs with out robust emission at 625 nm. Moreover, a bigger molar ratio of Ag+ to DNA is just not preferable [34].
Characterization of Apt76-AgNCs
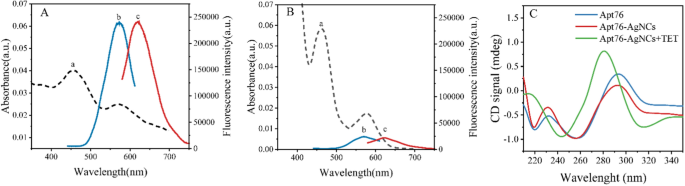
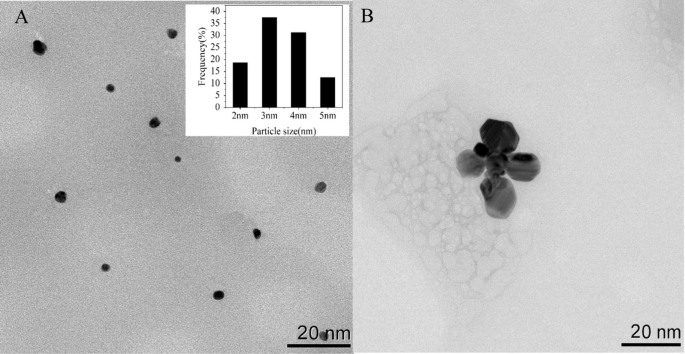
To analyze the optical traits of Apt76-AgNCs with out and with TET of 10 µg/mL, the UV–vis absorption and fluorescence spectra have been studied. There are two peaks at 450 and 570 nm in each UV–vis absorption spectra of Apt76-AgNCs alone (Fig. 3A, curve a) and with 10 µg/mL TET (Fig. 3B, curve a) possess, as illustrated in Fig. 3. The peaks at 450 and 570 nm correspond to a typical absorption band of Ag nanoparticles [34] and AgNCs [35], respectively. With 10 µg/mL TET current, the fluorescence depth at 625 nm of Apt76-AgNCs is weaker than Apt76-AgNCs alone, when excited at 570 nm (Fig. 3A, B, curve b and c), this is because of the truth that Apt76 folds right into a hairpin type within the presence of TET, altering the scale of AgNCs and inflicting fluorescence quenching. The Apt76-AgNCs have been characterised with Transmission electron microscopy (TEM). Apt76-AgNCs with out TET have a fairly homogeneous distribution, as illustrated in Fig. 4, and the particle measurement is generally 3 nm. Nonetheless, we are able to see intuitively and plainly that the presence of TET causes AgNCs aggregation and measurement enlargement, which explains why fluorescence traits of Apt76-AgNCs fluctuate with and with out TET.
Verification of the Apt76 conformation transition
In response to our research, the aptamer biosensor responded sensitively with the transition of AgNCs to AgNPs, and the conformation transition of Apt76 performed a task when TET was current. To substantiate the precept of the biosensor, additional verification experiments have been carried out. From the round dichroism (Fig. 3C), the unique Apt76 confirmed a unfavourable peak at round 260 nm and a constructive peak at roughly 290 nm, validating the properties of the aptamer’s G-quadruplex construction [36]. After the synthesis of AgNCs primarily based on Apt76, the height worth didn’t shift, however the CD sign diminished, which is likely to be attributable to the existence of Ag+ and Ag atoms affecting Apt76 stability to a sure extent [37]. After reacting with TET, the unfavourable peak of the CD spectrum of DNA-AgNCs shifted to roughly 245 nm, and the constructive peak moved to roughly 280 nm, that may be a typical CD spectrum function of B- sort DNA [38], marking the transition from the G-quadruplex to the hairpin construction transition [19].
Optimization of the experimental circumstances of the developed biosensor
The flexibility of Apt76-AgNCs to seize TET can also be a key think about figuring out the sensitivity of the designed biosensor. The decaying fluorescence indicators might be detected extra sensitively by assuring background fluorescence and boosting the response effectivity of Apt76 and TET as a lot as possible. Due to this fact, it’s essential to optimize the response buffer. Determine 5A exhibits the fluorescence values of Apt76-AgNCs with and with out TET and the fluorescence quenching effectivity of TET for various response buffers. It was reported that the optimum pH for the response between Apt76 and tetracycline was 7.6, so the pH of the buffers for use was managed at 7.6 [26]. The Tris-citrate buffer was ready in strict accordance with the ion focus of Apt76 screening buffer reported (5 mM Okay+,1 mM Ca2+,100 mM Na+,0.2 mM Mg2+,20 mM Tris), which not solely assured the fluorescence depth of AgNCs but in addition ensured the binding means of Apt76 to TET [26]. The buffer containing Cl– (PBS, Tris-HCl and Tris-hydrochloride) was discovered to have very low fluorescence emission as a result of Cl– may type AgCl with Ag+, which not directly quenched the fluorescence of AgNCs. Within the buffer answer with out metallic ions (Tris-citric acid), though it nonetheless possessed a excessive fluorescence emission, it didn’t have an excellent fluorescence quenching effectivity, which indicated that Apt76 wanted metallic ions to take care of the pocket form for capturing TET [39]. In PB buffer, the fluorescence quenching effectivity was decrease than that of Tris-citrate, probably because of the lack of divalent metallic cations. The hyperlink between the sign and response time was studied by including simply Apt76-AgNCs and TET to the optimum buffer to determine the suitable time to develop this biosensor. To get rid of interference produced by taking fixed measurements in a short interval, the answer was examined each 10 min. Throughout the first hundred minutes, the fluorescence depth quickly rose, as seen in Fig. 5B, after which leveled off. In consequence, the response period on this research was set at 100 min. Below the optimum circumstances, the Apt76-AgNCs have been ready, and the fluorescence spectra with totally different concentrations of TET in buffer have been measured. As seen in Fig. 5C, the absorption peaks at 625 nm diminished because the TET focus rose. In consequence, within the focus vary of 20 ng/mL–10 µg/mL was proven to have a powerful linear reference to the sign depth (R2 = 0.9977). The worth of LOD was 11.46 ng/mL and equated to a focus three commonplace deviations above the clean. Moreover, the recommended method is equal to these of the revealed biosensors for TET in Desk 1, as a consequence of its low LOD, simple operation, and fast detection time, in addition to the absence of chemical labeling.
A Influence of buffer on the fluorescence depth of Apt76-AgNCs and fluorescence quenching effectivity with TET. B Influence of the response time on the fluorescence quenching effectivity with out TET. C Detection of TET utilizing the developed biosensor. Fluorescence spectra with various TET doses, in addition to the biosensor’s response and analytical curves. D Selectivity of the sensor towards TET in opposition to different antibiotics (10 µg/mL). Three experiments resulted in error bars
Sensitivity and selectivity of TET detection
Specificity is a vital evaluation parameter for biosensor sensing efficiency. A number of antibiotics, together with penicillin, ciprofloxacin, chloramphenicol (CAP), streptomycin, ofloxacin and aminoglycosides (KANA), have been examined underneath optimum circumstances with 10 µg/mL. Solely TET could produce appreciable variation within the biosensor sign, as seen in Fig. 5D, and the associated fluorescence quenching effectivity is close to to 75%. Different antibiotics, then again, can not induce a substantial decline in biosensor sign. These findings present that the recommended fluorescent method has acceptable specificity for TET. The exceptional selectivity is because of the Apt76’s uncommon three-dimensional construction, which generates excessive affinity interactions with TET and may distinguish the TET and different antibiotics primarily based on minor structural variations.
Evaluation of TET in uncooked milk with the developed biosensor
The proposed sensor was used to check TET in milk to guage the dependability and applicability of the AgNCs biosensor in actual samples. The milk was pretreated in accordance with the approach outlined in earlier description. TET (20, 50, 100, 200, and 500 ng/mL) was added to the ultimate supernatant after which the biosensor was used to guage the spiked samples. As proven in Desk 2, the end result signifies that the recoveries of the spiked samples various from 97.7 to 114.6%, and the relative commonplace deviations (RSDs) are within the vary of two.8–8.5%. To additional validate the accuracy of the proposed methodology, the TET content material of the identical samples was measured by HPLC methodology. The outcomes obtained by the 2 strategies are constant in Desk 2. In consequence, the recommended biosensor presents a variety of functions in milk detection, with wonderful precision and accuracy.