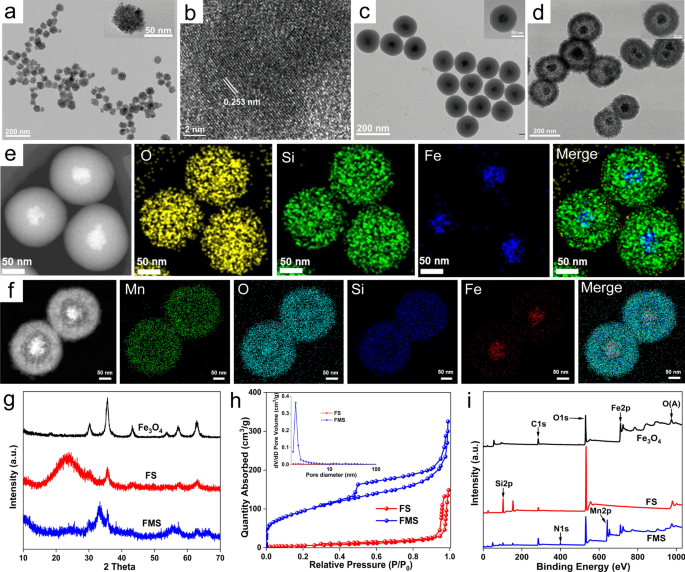
Synthesis of FMS yolk-shell nanoswitch
The preparation of FMS consisted of three steps, as illustrated in Scheme 1a. We first synthesized Fe3O4 nanoclusters by way of the solvent thermal decomposition technique utilizing ferric acetylacetonate because the iron supply and sodium acrylate because the template. As depicted in Fig. 1a, the Fe3O4 nanoclusters had been monodispersed and uniformly spherical with a dimension of roughly 60 nm. Furthermore, the hydrodynamic diameter of the Fe3O4 nanocluster was roughly 60 ± 19.7 nm, which is corresponding to the dimensions obtained from transmission electron microscopy (TEM) research, indicating that the Fe3O4 nanocluster had a superb colloidal efficiency. As well as, the high-resolution TEM (HRTEM) picture reveals that the Fe3O4 nanoclusters had been extremely crystalline, with a lattice fringe distance of ~ 0.253 nm, which corresponds to the (311) atomic aircraft of magnetite (Fig. 1b). Subsequently, the well-dispersed spherical Fe3O4@SiO2 (FS) core–shell nanoparticles with a shell thickness of ~ 35 ± 16.0 nm had been shaped by utilizing TEOS because the silica supply to coat the Fe3O4 below heating and alkaline circumstances (Fig. 1c). Lastly, a selected “ammonia-assisted Mn2+ etching” technique was used to etch the outer silica shell to kind Mn-doped silica-coated ferroferric oxide nanoparticles (FMS) by way of the Ostwald ripening mechanism [33]. In comparison with untreated FS nanoparticles, the consultant TEM picture revealed well-defined yolk-shell nanostructures with a bigger dimension (~ 220 ± 100.8 nm) (Fig. 1d). As well as, the FMS exhibited a porous construction that was free and tough, offering an abundance of lively websites for proton change. Moreover, the growing hydrodynamic dimension of nanoparticles from FS to FMS additionally confirmed the TEM observations (Extra file 1: Fig. S1). The high-angle annular darkish discipline scanning TEM (HAADF-STEM) picture of FS revealed the composition and nanostructure of the core–shell, however FMS additional illustated the profitable doping of Mn ions and nanostructure adjustments from core–shell to yolk-shell (Fig. 1e, f). Furthermore, the energy-dispersive X-ray (EDX) spectra confirmed that each one anticipated parts (Fe, Si, Mn, and O) had been detected and their relative positions in yolk-shell nanostructures are nicely matched (Extra file 1: Fig. S2g–i).
a TEM picture and b HRTEM picture of Fe3O4. TEM pictures of c FS and d FMS. HAADF-STEM and elemental distribution pictures of e FS and f FMS. g XRD patterns of Fe3O4, FS, and FMS. h N2 adsorption/desorption isotherms of FS and FMS (inset: pore dimension distribution), i XPS full spectra of Fe3O4, FS, and FMS
As well as, X-ray diffraction (XRD) spectra of Fe3O4 revealed the attribute spinel construction of magnetite (Fig. 1g) [34]. Upon progress of the SiO2 shell layer, a attribute broad peak of amorphous silica is produced, whereas it almost disappears after Mn2+ ions etch the FMS. This consequence signifies that the silica shell is efficiently coated on the floor of Fe3O4 nanoparticles, and it’s successfully etched by Mn2+ within the subsequent course of. After Mn2+ etching, important will increase in particular floor space (342.92 m2/g) and porosity are noticed for FMS nanostructures with a pore dimension distribution dominated by 2.0 nm (Fig. 1h). As is well-known, the load lack of the thermogravimetric (TG) curves beneath 200 °C is attributable to the lack of water molecules, together with bodily adsorbed and sure water. Accordingly, the TG evaluation means that FMS adsorbs considerably extra water (7.98%) than FS (6.58%) and Fe3O4 (2.35%) (Extra file 1: Fig. S2a). These findings point out that FMS has a robust affinity for water molecules and facilitates the interplay with the proton, which might enhance the relief properties.
Within the Fourier rework infrared (FT-IR) spectrum of Fe3O4, the height of the Fe–O bond seems at 570 cm−1 [34], whereas the standard peaks of the –C=O and C–H bonds seem at 1635 cm−1 and 2920 cm–1, respectively (Extra file 1: Fig. S2e). As well as, the FT-IR spectrum of FS displays a definite Si–O peak at 1100 cm−1 [35], indicating that silica has been efficiently coated on the floor of Fe3O4. Furthermore, the Si–O peak of FMS shifts considerably from 1100 to 1000 cm−1 because of the transformation of the Si–O-Si bond to the Mn–O-Si bond, indicating the profitable etching of the silica layer. Within the PDGFB-FMS FT-IR curve, the brand new peak at 1450 cm−1 signifies that PDGFB is efficiently anchored to the floor of FMS. The formation of PDGFB-FMS nanoswitch is additional supported by ζ potential adjustments of particles (Extra file 1: Fig. S2f). The outer SiO2 layer of the FS displays a unfavourable ζ potential of roughly − 46.3 mV. In distinction, the ζ potential of FMS decreased to − 15.5 mV, confirming the presence of Mn2+ ions. Moreover, the ζ potential of PDGFB-FMS was discovered to be − 18 mV, indicating that the conjugation of PDGFB was profitable. The presence of Fe, Si, Mn, C, and O was additional confirmed from the complete XPS spectrum of FMS (Fig. 1i). Because of the dense, thick SiO2 shell that obscured the Fe sign within the Fe2p high-resolution spectrum of FS, Fe2p peaks at 711.8 and 724.3 eV vanished. Notably, the Fe peaks of the FMS yolk-shell nanostructure recovered, and powerful Mn peaks emerged (Fig. 1i and Extra file 1: Fig. S2b, d), indicating the formation of a free Mn-doped silica shell layer. Remarkably, the Si2p peak for FS shifted considerably from 103.3 to 102.4 eV (Extra file 1: Fig. S2c), additional confirming the transformation of Si from a dense silica layer to a free construction. The hydrodynamic dimension of PDGFB-FMS had no important variation in blood, phosphate-buffered saline (PBS), and 10% fetal bovine serum (FBS) inside 3 days, demonstating glorious stability (Extra file 1: Fig. S3).
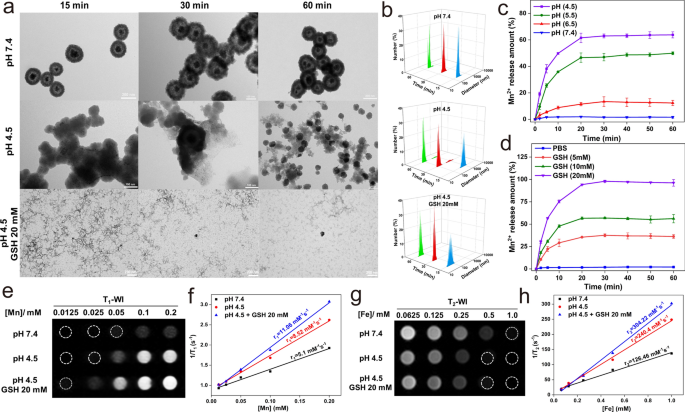
TME-responsive “off–on” T1–T2 imaging of FMS nanoswitch
We additionally analyzed the degradation of FMS below GSH and weak acid circumstances. Within the presence of weak acidity and GSH, Fig. 2a, b illustrates the numerous results of FMS on the hydrodynamic dimension distribution and morphology of corresponding particles. Most FMS particles disassemble from common spherical buildings into scattered dots over time. In distinction, no discernible change is noticed for FMS within the absence of GSH and below impartial circumstances. As well as, the diploma of dissociation of FMS elevated considerably with growing GSH focus and lowering pH, demonstrating glorious pH- and GSH-responsive degradability (Extra file 1: Fig. S4). To achieve extra perception into the evolution course of, the discharge of Mn2+ ions from FMS with the change of GSH focus and pH worth was investigated. Accordingly, the outcomes verify that the habits of Mn2+ ion launch from FMS is pH- and GSH-dependent (Fig. 2c, d). These outcomes point out that FMS could be responsively dissociated in TME with an abundance of GSH and an acidic atmosphere by breaking the Mn–O bond and the following collapse of the FMS spine inside one hour (Fig. 2c, d). Based mostly on the wonderful degradation of FMS triggered by TME, we additional investigated the off–on switchable properties of the dual-mode T1–T2 sign in FMS. Underneath physiological circumstances (in pH 7.4 PBS), each T1– and T2-weighted imaging (T1WI and T2WI, respectively) exhibited an obvious quenching phenomenon (Fig. 2e, g). As well as, the corresponding T1 relaxivity (r1) and T2 relaxivity (r2) had been measured to be 5.1 and 126.46 mM−1 s−1, respectively (Fig. 2f, h). When uncovered to GSH (20 mM) and pH 4.5, the FMS nanostructure decomposed quickly into Fe3O4 and Mn2+ ions, leading to a fast restoration of T1 and T2 indicators as the gap between the Mn2+ ions and Fe3O4 magnetic core elevated (Fig. 2e, g). After therapy with PBS containing 20 mM GSH and pH 4.5, the calculated r1 and r2 values had been discovered to be 11.06 and 304.22 mM−1 s−1, respectively (Fig. 2f, h). As well as, as depicted in Extra file 1: Figs. S5, S6, the “off–on” traits of dual-mode T1–T2 nanoprobes had been extremely depending on GSH concentrations and pH values. These outcomes, subsequently, assist our speculation that low pH and excessive GSH concentrations triggered the discharge of Mn2+, which reduces the mutual magnetic resonance interference between Mn2+ and Fe3O4 and prompts the T1–T2 MRI indicators (Extra file 1: Tables S1 and S2).
a TEM pictures and b corresponding hydrodynamic dimension adjustments (blue, 15 min; crimson, 30 min; inexperienced, 60 min) of FMS after handled with completely different pHs and GSH circumstances. c, d Cumulative Mn2+ launch curves of FMS at completely different c pH and d GSH concentrations. e T1WI and f corresponding r1 values of the FMS phantoms after completely different therapies below 3.0 T. g T2WI and h corresponding r2 values of the FMS phantoms after completely different therapies below 3.0 T
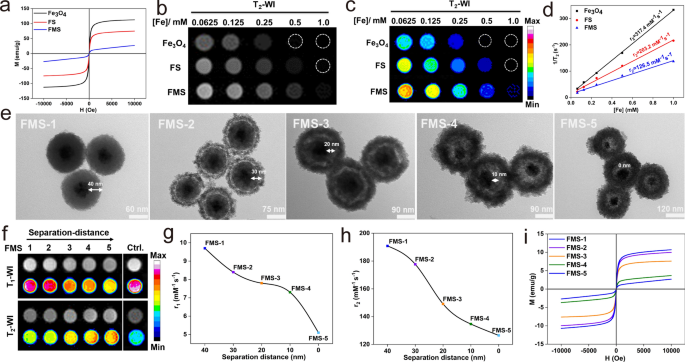
Quenching mechanism of T1 and T2 imaging
The T1–T2 dual-quenching mechanism of FMS was additionally investigated methodically. Accordingly, Mn2+ ions with a single T1 distinction and Fe3O4 with a single T2 distinction had been used as controls (Extra file 1: Fig. S7a and Fig. 3). For the T1 quenching impact, we proposed that the robust native magnetic discipline originating from the big magnetic second of the Fe3O4 magnetic core would intervene with the spin–lattice rest strategy of Mn-doped silica shell with a lot weaker paramagnetism surrounding it, thereby quenching the T1 sign of paramagnetic Mn2+. Accordingly, the M-H curves of FMS exhibited a decrease saturation magnetization (25 emu/g) at 300 Okay compared to Fe3O4 (112.4 emu/g). As well as, the magnetic discipline generated by the superparamagnetic Fe3O4 core considerably disrupts the relief strategy of the paramagnetic Mn-doped silica shell, ensuing within the quenching of the T1 sign in the course of the etching process (Fig. 3a). In the meantime, the grayscale and corresponding pseudo-color T2WI of Fe3O4, FS, and FMS exhibited important T2 quenching results (Fig. 3b, c), and their T2 relaxivities (r2) had been 317.4 mM−1 s−1, 203.2 mM−1 s−1, and 126.5 mM−1 s−1, respectively (Fig. 3d). Subsequently, T1 and T2 MRI indicators could be interfered with when Fe3O4 and Mn-doped silica are in direct contact. To additional validate the MRET speculation, we synthesized a collection of FMS (FMS-1, FMS-2, FMS-3, FMS-4, FMS-5) with completely different separation distances between the paramagnetic Mn2+ and the Fe3O4 magnetic core by various the thickness of the silica separation layer from 40 ± 2.6 to 0 ± 1.2 nm (Fig. 3e). Notably, the hydrodynamic dimension of FMS elevated regularly because the etching course of progressed (Extra file 1: Fig. S7b), whereas concurrently growing the particular floor space and porosity (Extra file 1: Fig. S8), facilitating the power change with water molecules. The nanoswitch confirmed apparent quenching in T1 and T2 indicators with the Mn2+ doping ratio from 33 to 88% (Extra file 1: Fig. S9). This was as a result of that prime ratio of Mn-doped silica considerably lowered the gap between Fe3O4 core and Mn2+, ensuing within the improve of quenching impact in T1 and T2 imaging. As proven in Fig. 3f, the T1 MRI sign of the Mn-doped silica shell in FMS decreases because the Mn approaches the Fe3O4 core. Moreover, because the separation distance decreases from 40 to 30, 20, 10, and 0 nm, the r1 values of the Mn-doped silica shell lower from 9.7 to eight.4, 7.8, 7.3, and 5.1 mM−1 s−1 (Fig. 3g and Extra file 1: Fig. S7c), which is decrease than that of free Mn2+ (r1 = 15.9 mM−1 s−1) (Extra file 1: Fig. S7a). Concurrently, the same T2 MRI sign quenching phenomenon was noticed in T2WI and corresponding pseudo-color MR pictures (Fig. 3f). Because the etching depth will increase, the r2 worth of Fe3O4 decreases from 190.85 to 177.66, 149.15, 134.67, and 126.5 mM−1 s−1 (Fig. 3h and Extra file 1: Fig. S7d). As well as, the saturated magnetization of FMS decreased regularly with growing Mn content material, which could possibly be attributed to the disruption of Fe3O4 magnetic moments induced by paramagnetic Mn2+ (Fig. 3i). Thus, the aforementioned outcomes exhibit that the T1 and T2 dual-quenching impact of FMS is inversely proportional to the separation distance, which could be exactly managed by the Mn etching course of.
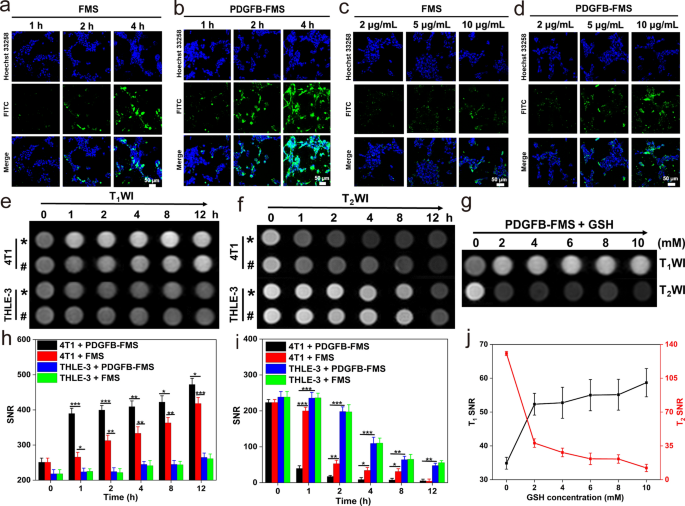
Analysis of internalization and cell MRI of PDGFB-FMS
The hypoxic TME stimulates the overexpression of PDGF-β receptors in breast most cancers cells and prostate most cancers cells [36, 37]. To acquire tumor-specific supply, the PDGFB focusing on ligand is, subsequently, conjugated to FMS to create a PDGFB-FMS nanoswitch that targets tumors. The internalization efficacy of a nanoprobe is important for evaluating its focusing on functionality and distinction efficiency. Utilizing confocal laser scanning microscopy (CLSM) and inductively coupled plasma mass spectrometry (ICP-MS) evaluation, the uptake of FMS and PDGFB-FMS was, subsequently, investigated in 4T1 cells and PC-3 cells based mostly on this info (Fig. 4a–d, and Extra file 1: Figs. S10, S11). The outcomes of CLSM indicated that the internalization of FMS and PDGFB-FMS is concentration- and time-dependent. As well as, PDGFB-FMS handled cells exhibited a stronger inexperienced fluorescence than FMS-treated cells, confirming their superior focusing on means to breast most cancers and prostate most cancers. We additionally calculated the colocalization and particle uptake ratios equivalent to the CLSM pictures. We discovered that the variations in cell internalization for FMS and PDGFB-FMS therapies are statistically important (Extra file 1: Fig. S12). As well as, Fe content material was used as an indicator for ICP-MS evaluation to find out the internalization efficacy of PDGFB-FMS. Outcomes point out that Fe content material in 4T1 cells elevated regularly with growing time and focus, and cells handled with PDGFB-FMS gathered extra Fe than these handled with FMS. These outcomes are in keeping with the CLSM statement, additional validating the efficiency of PDGFB-FMS in focusing on tumors.
a, b CLSM statement: the internalization strategy of 4T1 cells handled with FMS and PDGFB-FMS at a sure focus of 10 μg/mL for 1, 2, and 4 h. c, d CLSM statement: the internalization strategy of 4T1 cells handled with completely different concentrations of FMS and PDGFB-FMS for 4 h. e T1WI and f T2WI of the cells handled with completely different samples (* and # symbolize PDGFB-FMS and FMS, respectively) at completely different time factors, and h, i the corresponding T1 and T2 MRI SNR evaluation. g T1WI and T2WI of 4T1 cells handled with PDGFB-FMS on the presence of various concentrations of GSH, and j the corresponding T1 and T2 MRI SNR evaluation
The imaging efficiency of PDGFB-FMS was additional investigated on the mobile stage, in gentle of the aforementioned encouraging findings. The T1 and T2-weighted indicators of 4T1 cells handled with FMS and PDGFB-FMS had been discovered to be considerably activated (Fig. 4e, f, h, i) and exhibit a time-activation relationship. Notably, the T1 and T2 sign virtually remained unchanged in THLE-3 cells incubated with FMS and PDGFB-FMS, which could be attributed to the decrease mobile GSH stage and elevated internalization efficacy. To find out whether or not the activation of PDGFB-FMS correlates positively with intracellular GSH ranges, 4T1 cells had been pretreated with varied concentrations of GSH after which incubated constantly with PDGFB-FMS. The outcomes revealed that each the T1 and T2 indicators of cells had been considerably improved (Fig. 4g, j). Furthermore, the corresponding rest fee change ΔR1 (R1 = 1/T1) and ΔR2 (R2 = 1/T2) elevated regularly after GSH pretreatment (Extra file 1: Desk S3), suggesting that the intracellular excessive stage of GSH can be utilized as a stimulus to activate the dual-mode MRI distinction enhancement.
In vivo MRI research of PDGFB-FMS
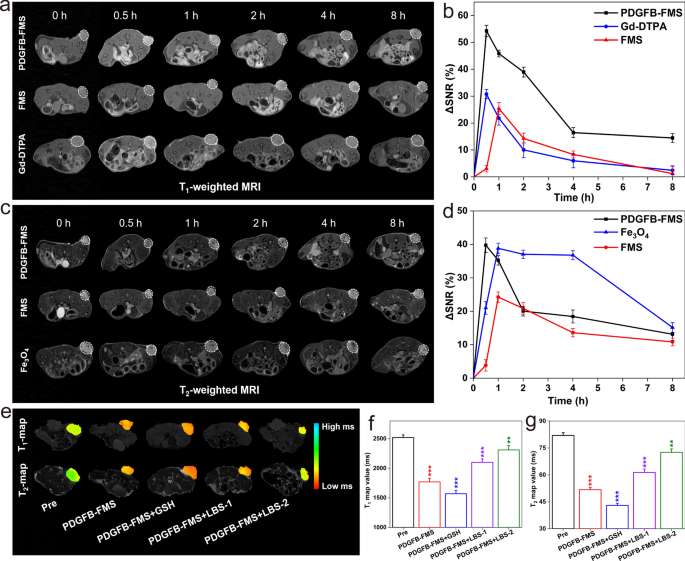
A collection of in vivo MRI experiments had been additionally designed and carried out utilizing a mouse mannequin bearing the 4T1 tumor to validate the activable MRI prognosis for the tumor. FDA-approved “Magnevist” (Gd-DTPA) and “Feridex” (Fe3O4) had been chosen as controls for T1WI and T2WI, respectively. Accordingly, the 4T1 tumor-bearing mice had been intravenously administrated utilizing Gd-DTPA, Fe3O4, FMS, and PDGFB-FMS on the dosage of 5 mg/kg, respectively, following which, they had been scanned utilizing a 7.0 T MRI scanner, and the T1WI and T2WI of the tumor within the axial aircraft had been obtained at varied time factors. As proven in Fig. 5a, the T1WI of tumors handled with PDGFB-FMS brightens considerably at 0.5 h post-injection (p.i.) after which darkens over time. As well as, the tumor web site of the PDGFB-FMS group in T1WI was noticed to be the clearest and brightest in comparison with these of FMS and Gd-DTPA. Equally to T1WI, the T2WI of the tumor handled with PDGFB-FMS was noticed to be darkest at p.i. 0.5 h (Fig. 5c), following the identical sample as T1WI. Quite the opposite, the distinction enhancement of FDA-approved Gd-DTPA and Fe3O4 for T1WI and T2WI, respectively, was discovered to be weak. As well as, the quantitative evaluation of tumor areas is carried out by measuring the change in SNR (ΔSNR) earlier than and after administration. As proven in Fig. 5b, the utmost ΔSNR of the tumor areas within the PDGFB-FMS group was higher than that of the FMS and Gd-DTPA teams, demonstrating probably the most pronounced distinction enhancement. As well as, T2WI of mice handled with FMS exhibited a low ΔSNR, whereas, PDGFB-FMS exhibited a comparable ΔSNR (Fig. 5d), which can be a results of environment friendly focusing on. As well as, the respective T1 and T2 map pictures confirmed the aforementioned findings. As proven in Extra file 1: Fig. S13, there was a 30.7% lower within the T1 map worth within the tumor area handled with PDGFB-FMS at p.i. 0.5 h, whereas the Gd-DTPA and FMS teams solely led to a 16.5% and 16.6% lower, respectively. Equally, a big lower within the T2 map worth is noticed at p.i. 0.5 h, and it follows the identical sample because the T1 map worth. The aforementioned outcomes exhibit that the PDGFB-FMS dual-activated nanoswitch has the specified dual-modal imaging functionality for correct tumor prognosis.
a, c Consultant T1WI and T2WI of 4T1 tumor-bearing mouse at axial planes after intravenous injection of various samples and b, d ∆SNR evaluation equivalent to a, c, respectively. Tumor area are marked by white dotted traces. e color-coded T1 and T2 map pictures, f corresponding T1 map worth, and g T2 map values of 4T1 tumor-bearing mice handled with PDGFB-FMS on the presence of GSH or GSH inhibitor (LBS)
To additional illustrate GSH tuning nanoswitch, we examined the T1 and T2 tumor map pictures following PDGFB-FMS therapy within the absence and presence of GSH and GSH inhibitor (LBS). Briefly, mice carrying 4T1 tumors had been intratumorally pretreated with 10 mM GSH, 2.5 mM LBS (LBS-1), or 5 mM LBS (LBS-2), respectively. The PDGFB-FMS was then administered intravenously to those 4T1 tumor-bearing mice. As anticipated, the GSH-pretreated tumor exhibited decrease T1 and T2 map values on the identical PDGFB-FMS injection as in comparison with the non-GSH-pretreated tumor. In distinction, T1 and T2 map worth attenuation of the tumor after LBS pretreatment was considerably inhibited, and their inhibition impact was discovered to be concentration-dependent (Fig. 5e–g). This phenomenon signifies that the decreased intratumoral GSH focus attributable to LBS prevented the activation of GSH-activated T1–T2 indicators. The findings, subsequently, clearly exhibit the clear optimistic correlation between PDGFB-FMS activation and intracellular GSH concentrations.
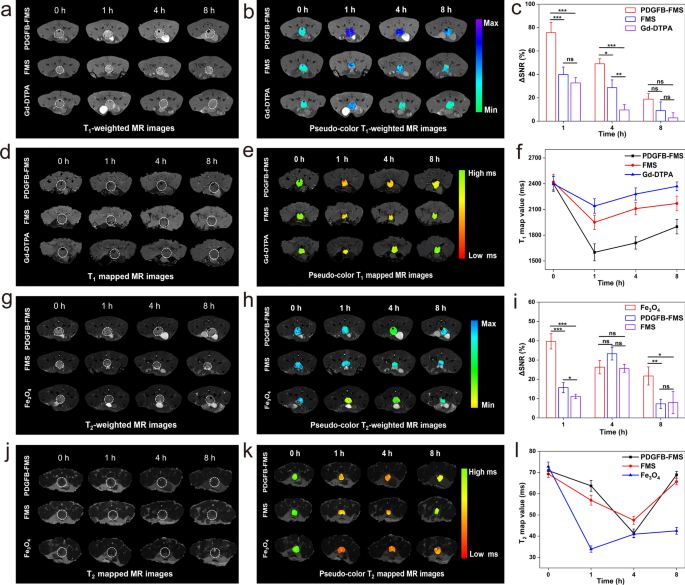
The imaging potential of the PDGFB-FMS nanoswitch for in-situ carcinoma was additionally investigated (Fig. 6). This examine establishes the transgenic adenocarcinoma of mouse prostatic (TRAMP) mannequin to simulate the development of orthotopic prostate most cancers [38, 39]. We first assessed the cytotoxicity of PDGFB-FMS nanoswitch by way of cell counting kit-8 (CCK-8) assays earlier than in vivo MRI research. No obvious cytotoxicity towards PC-3 cells was noticed for PDGFB-FMS nanoswitch, indicating that PDGFB-FMS nanoswitch has good biocompatibility (Extra file 1: Fig. S14). Then, transgenic adenocarcinoma mouse prostate (TRAMP) mice had been administered Gd-DTPA, Fe3O4, FMS, and PDGFB-FMS intravenously, respectively. Correspondingly, the T1 and T2 MR contrasts of the prostate tumor web site had been considerably enhanced 1 and 4 h post-injection of PDGFB-FMS, respectively. Nevertheless, the modest dynamic distinction enhancement of T1 and T2 within the Gd-DTPA, Fe3O4, and FMS teams was seen 8 h after injection (Fig. 6a, b, g, h). The ΔSNR was then used to quantify the MR indicators of tumors at completely different time factors (Fig. 6c, i). Accordingly, for T1-weighted MRI, the utmost ΔSNR of tumors handled with PDGFB-FMS reached as much as 75.6 ± 8.8%, which was considerably higher than that of tumors handled with FMS (39.9 ± 6.3%) and Gd-DTPA (32.7 ± 4.5%) (Fig. 6c). Utilizing T2-weighted MRI, the maximal ΔSNR of tumors handled with PDGFB-FMS, FMS, and Fe3O4 is 33.3%, 25.7%, and 39.7%, respectively (Fig. 6i). These outcomes recommend that PDGFB-FMS targets tumor websites and that T1 and T2 imaging are successfully illuminated by TME. Moreover, we collected T1 and T2 map pictures to confirm this conclusion (Fig. 6d, e, j, and ok). On the tumor handled with PDGFB-FMS, roughly 33.3% of discount in T1 map worth was detected, whereas FM and Gd-DTPA solely led to 19.4% and 10.8% discount in T1 map worth, respectively (Fig. 6f). Furthermore, PDGFB-FMS exhibited a big lower in T2 map worth (41.7% at p.i. 4 h), whereas T2 map worth adjustments for FMS and Fe3O4 had been 31.9% and 53.0%, respectively (Fig. 6l). Contemplating the considerably greater T1 and T2 ΔSNR and the delicate “off–on” dual-mode MRI indicators, the PDGFB-FMS nanoswitch demonstrates its superiority within the space of early-stage most cancers detection.
MRI visualization of early orthotopic prostate tumor-bearing mice handled with completely different samples. a Grayscale and b pseudocolor T1WI. c ΔSNR equivalent to a. d Grayscale and e color-coded T1 map pictures. f T1 map values evaluation equivalent to d. g Grayscale and h pseudocolor T2WI. i ΔSNR equivalent to g. j Grayscale and ok color-coded T2 map pictures. l T2 map values evaluation equivalent to j. Tumor areas are indicated by white dotted traces
In vivo biosafety evaluation of PDGFB-FMS nanoswitch
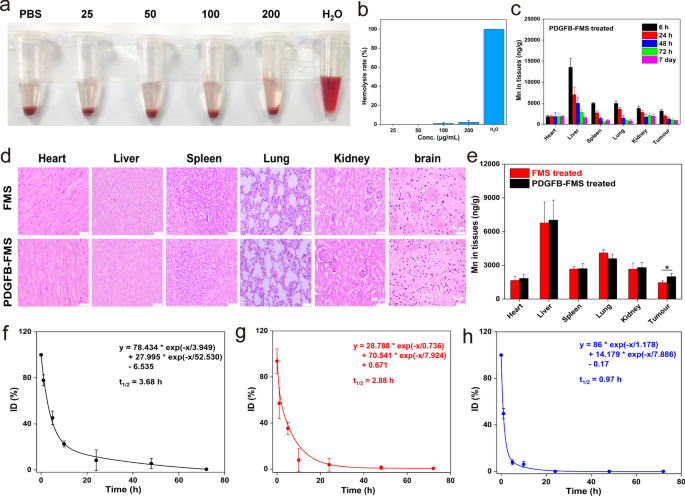
As is well-known, the biosafety of a nanoagent is an important issue to contemplate when assessing its scientific utility potential [40, 41]. Based mostly on this, we first consider the biocompatibility of PDGFB-FMS with blood utilizing a hemolysis assay and a routine blood examination. As proven in Fig. 7a and b, the hemolysis fee of PDGFB-FMS is extraordinarily low and negligible, indicating that PDGFB-FMS can not injury crimson cells. As well as, main blood routine indicators resembling WBC, RBC, PLT, HGB, and HCT (Extra file 1: Fig. S15) at p.i. PDGFB-FMS didn’t exhibit any important adjustments. These outcomes exhibit the biocompatibility of PDGFB-FMS with blood. Subsequently, we additionally investigated cytoxicity of PDGFB-FMS on regular cells (THLE-3, 293T, PC-12, and SH-SY5Y cells). As proven in Extra file 1: Fig. S16a–c, the PDGFB-FMS is biocompatible with THLE-3, 293T, PC-12, and SH-SY5Y cells over a variety of concentrations, exhibiting no toxicity. We additionally explored tissue toxicity of PDGFB-FMS utilizing hematoxylin and eosin (H&E) evaluation. After 7 days of PDGFB-FMS therapy, no apparent abnormalities within the important organs had been noticed, confirming the wonderful biosafety of the tissue (Fig. 7d). As well as, the biodistribution evaluation revealed that the reticuloendothelial system was accountable for almost all of PDGFB-FMS accumulation within the liver (Fig. 7c). Notably, PDGFB-FMS ranges within the physique decreased considerably over time, indicating that it may be excreted successfully. Apart from the buildup within the liver on the early levels, PDGFB-FMS reveals greater accumulation in tumors than non-targeted FMS, demonstrating glorious tumor-targeting (Fig. 7e). Additional analysis was additionally carried out into the pharmacokinetics of PDGFB-FMS, FMS, and Gd-DTPA. At p.i. 10 h, the residual concentrations of FMS and Gd-DTPA had been solely 18.9% and 4.6%, respectively, whereas PDGFB-FMS was 23.8%. Furthermore, PDGFB-FMS had the longest half-life within the blood (t1/2 = 3.68 h) in comparison with FMS (t1/2 = 2.88 h) and Gd-DTPA (t1/2 = 0.97 h) (Fig. 7f–h). Notably, at p.i. 72 h, PDGFB-FMS could possibly be utterly eradicated from the physique, thereby avoiding the potential dangers to the physique posed by long-term residual [42]. To summarize, the superior biocompatibility of PDGFB-FMS and its immediate in vivo clearance facilitate its use in tumor molecular MRI.
a Hemolysis pictures and b hemolysis fee of PDGFB-FMS. c Bio-distribution of Mn ingredient after i.v. injection of PDGFB-FMS. d H&E staining of main organs within the mice handled with FMS and PDGFB-FMS for 7 days. The dimensions is 50 μm. e Bio-distributions of Mn ingredient in main organs after handled with PDGFB-FMS and FMS for twenty-four h. Pharmacokinetic curves of f PDGFB-FMS, g FMS, and h Gd-DTPA in vivo