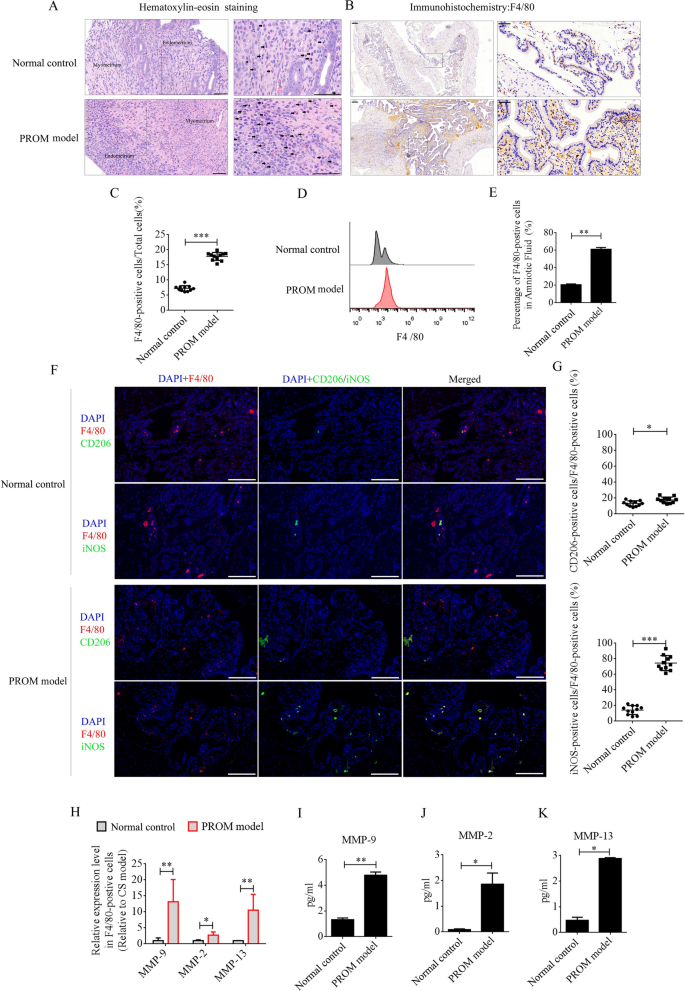
Macrophage recruitment and polarization into the M1 phenotype in PROM mice
LPS was intraperitoneally injected into mice at day 15 of being pregnant to ascertain the PROM mannequin. PROM occurred at roughly 48 h. Maternal macrophages are regularly enriched within the decidua as being pregnant progresses [12]. LPS, as a classical inflammatory inducer of macrophages, promotes polarization to the M1 phenotype. Numerous inflammatory components are then produced and secreted from the M1 macrophages, together with MMPs, which speed up collagen degradation and cervix softening to induce PROM. On this examine, we first in contrast the recruitment and polarization of macrophages in uterine tissues and amniotic fluid between PROM mannequin and pregnant regular management mice at day 17 of being pregnant. Underneath hematoxylin–eosin (HE) staining, monocytes had been noticed within the decidua of the uterus in each the PROM and regular management mice, and the numbers had been discovered to be dramatically elevated in contrast with these in PROM mannequin (Fig. 1A). F4/80 is the principle marker of macrophages in several tissues, and immunohistochemistry (IHC) of uterine tissue revealed that F4/80-positive cells had been expressed at considerably increased ranges within the PROM mannequin in contrast with these within the regular management (p < 0.001, Fig. 1B and 1C). Moreover, the share of F4/80-positive cells in amniotic fluid was detected by circulate cytometry. In settlement with the IHC information, the share of F4/80-positive cells in amniotic fluid from the PROM mannequin was increased than in that from the conventional management (p < 0.01, Fig. 1D and 1E). Subsequent, F4/80-positive cells in uteri from PROM and regular management mice had been analyzed by immunofluorescence for M1 or M2 phenotype polarization utilizing inducible nitric oxide synthase (iNOS) or CD206 antibodies. The information revealed that nearly 80% of F4/80-positive cells within the PROM mannequin expressed iNOS, which was considerably increased than within the regular management (p < 0.05, Fig. 1F and G). These information steered that M1 macrophages play a crucial position in PROM. Subsequent, F4/80-positive cells in amniotic fluid had been chosen by magnetic cell separation and used to detect mRNA expression of MMP genes by real-time PCR. Expression ranges of Mmp9, Mmp2, and Mmp13 mRNA had been dramatically modified in F4/80-positive cells derived from the PROM mannequin in contrast with these in cells from the conventional management (Fig. 1H). Protein expression of MMP-9, MMP-2, and MMP-13 in amniotic fluid was analyzed by enzyme-linked immunosorbent assay (ELISA), which revealed that the degrees had been markedly elevated within the PROM mannequin (p < 0.05, Fig. 1I–Okay).
Recruitment and polarization of macrophages within the uterus of PROM mannequin and regular management mice. A HE staining to research monocyte numbers in the identical interval in gestational uterus derived from regular management and PROM mannequin mice. Consultant monocytes are indicated with black arrows. B and C Anti-F4/80 antibody evaluation and quantification of the variety of macrophages in uteri. D and E Move cytometry and counting of macrophages in amniotic fluid. F Consultant immunofluorescence photographs of iNOS- or CD206-positive cells within the uteri. G Quantification of iNOS- and CD206-positive cells within the uteri. Scale bar = 100 μm. H mRNA ranges of MMPs in F4/80-positive cells derived from regular management and PROM mannequin mice. I, J and Okay ELISA detection of MMP-9, MMP-2, and MMP-13 expression in amniotic fluid; *p < 0.05; **p < 0.01; ***p < 0.001
CircRNA appearing as a miRNA sponge maintains polarization of macrophages and MMP secretion
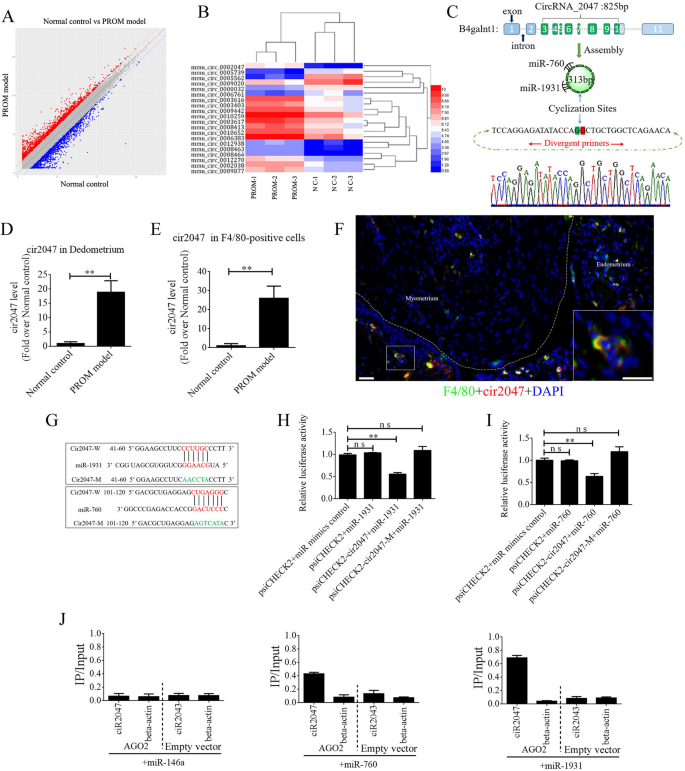
The ceRNAs are broadly reported to perform in lots of organic processes and in illness incidence. To exclude the underlying mechanism of ceRNA in sustaining polarization of macrophages, we peeled the decidua from the uterine lining and remoted complete RNA to carry out ceRNA profiling. Roughly 1000 circRNAs exhibited a major change of their expression stage between the PROM mannequin and regular management (Fig. 2A). A warmth map of the highest 20 circRNAs is proven in Fig. 2B. Bioinformatics evaluation revealed that mmu_circRNA_0002047 (cir2047, ENSMUST00000006914) has potential binding websites for miR-1931 and miR-760, and was ranked among the many most importantly modified genes within the evaluation. Cir2047 has eight exons overlaying 313 bp and is expressed by the host gene B4galnt1. To confirm the ceRNA profiling information, we first confirmed a major change within the stage of cir2047 within the decidua by inspecting F4/80-positive cells from amniotic fluid, earlier than and after LPS remedy, utilizing real-time PCR and product sequencing. The extent of cir2047 was elevated dramatically after LPS remedy (p < 0.01, Fig. 2C–E). To verify cir2047 expression in naïve macrophages within the uterus, in situ hybridization and IHC had been carried out following LPS remedy. The outcomes demonstrated that cir2047 and F4/80 had been co-expressed in naïve macrophages (Fig. 2F). To research the interplay of cir2047 and miR-1931 or miR-760, the miRNA binding websites in cir2047 had been mutated by PCR (cir2047-mut) after which cloned right into a luciferase reporter system, together with wild-type cir2047 (cir2047-wt) as a optimistic management. These constructs had been co-transfected into F4/80-positive cells with both a miRNA progenitor or a management. In F4/80-positive cells co-transfected with the miRNA progenitor and cir2047-wt, luciferase exercise was dramatically decreased relative to cells co-transfected with the miRNA progenitor and cir2047-mut (p < 0.05, Fig. 2G–I). Argonaute 2 (AGO2) protein is a vital element of the RNA-induced silencing complicated that performs a central position in miRNA silencing. To verify whether or not AGO2 serves as a binding platform for miR-1931 and cir2047, we carried out AGO2 immunoprecipitation (IP) in F4/80-positive cells carrying both an AGO2 expression vector or empty vector, and transiently co-expressed miR-1931, miR-760, or miR-146a-5p as a destructive management (there isn’t a miR-146a-5p binding website within the cir2047 sequence). Actual-time PCR was used to research the extent of cir2047 in IP merchandise. Cir2047 was particularly enriched by greater than six- and four-fold within the presence of AGO2 in miR-1931– and miR-760-transfected cells, respectively, in contrast with that within the destructive management (miR-146a-transfected cells; Fig. 2J).
Adjustments in CircRNAs within the uteri of regular management (NC) and PROM mannequin mice. A CeRNA profiling exhibiting important modifications in circRNA expression within the uteri between NC and PROM mannequin mice. B Warmth map of the highest 20 circRNAs exhibiting important modifications following PROM incidence. C Schematic illustration of cir2047 construction and sponged miR-760-5p and miR-1931. D and E Expression of cir2047 measured by qPCR within the decidua and F4/80-positive cells. F Co-expression of F4/80 and cir2047 in uterine tissue detected by in situ hybridization and IF. Scale bar = 50 μm. G Binding websites of cir2047 and miRNAs had been predicted by bioinformatics algorithms and subsequently mutated to confirm interactions. H and I Results of cir2047-sponged miR-1931 and miR-760 had been validated utilizing luciferase reporter vector assays. Full-length cir2047 and sequences containing completely different mutated variants of the miRNA-binding website (cir2047-M) had been used to detect the suppressed results of miR-1931 and miR-760. J IP of AGO2 from F4/80-positive cells co-transfected with AGO2 and both miR-1931, miR-760, or miR-146a (destructive management). Empty vector served because the AGO2-related destructive management. Cir2047 and ACTB mRNA ranges had been quantified by qPCR and relative IP/enter (complete mobile RNA) values had been plotted; **p < 0.01; ns, not important
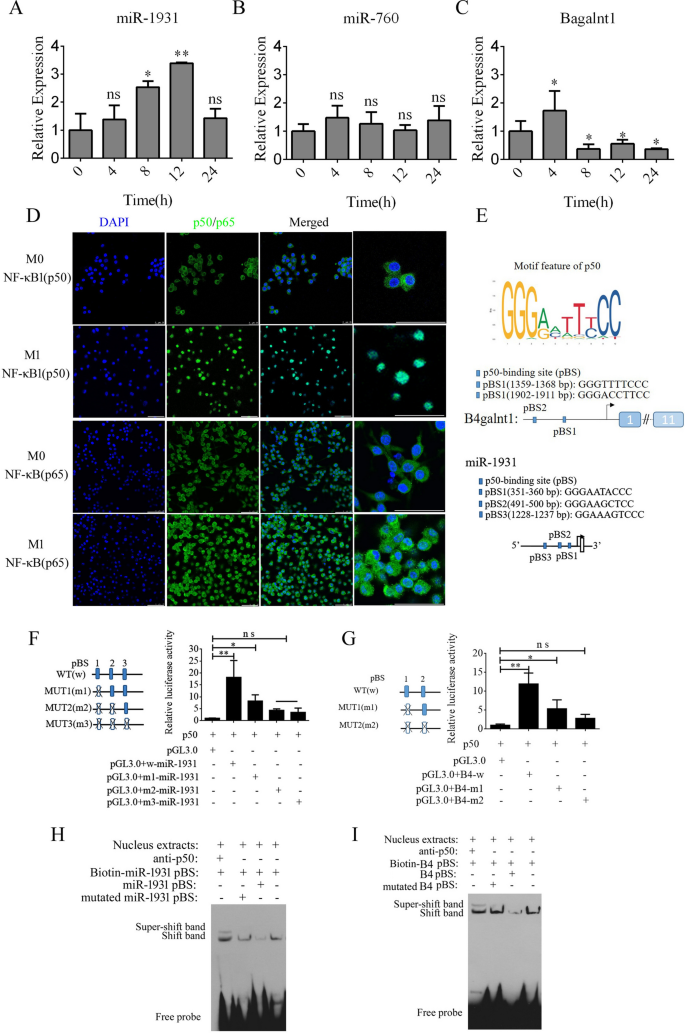
By analyzing the expression of B4galnt1, miR-1931, and miR-760 in F4/80-positive cells utilizing real-time PCR, we discovered that B4galnt1 and miR-1931 had been transiently upregulated following LPS and interferon (IFN)-γ remedy in F4/80-positive cells, however miR-760 didn’t endure a major change (Fig. 3A–C). These information implied that the linear mRNA of B4galnt1 was circularized to type a circRNA that sponged miR-1931 in F4/80-positive cells after LPS and IFN-γ remedy. The NF-κB pathway performs an necessary position within the polarization of M1 macrophages and within the manufacturing of pro-inflammatory components. NF-κB is a dimer of the Rel household of 5 proteins, and a heterodimer of NF-κB (p65) and NF-κB1 (p50) subunits was the primary described NF-κB molecule. We detected NF-κB (p65) and NF-κB1 (p50) by immunofluorescence in F4/80-positive cells following M1 polarization. In Fig. 3D, NF-κB1 (p50) had virtually entered the nucleus, whereas some NF-κB (p65) remained within the cytoplasm. Bioinformatics evaluation of the promoter areas of B4galnt1 and miR-1931 revealed that they’re NF-κB pathway-dependent genes with promotor areas that bind NF-κB1 (p50) (Fig. 3E). To check the regulation of binding websites by NF-κB1 (p50), the binding websites for NF-κB1 (p50) had been mutated individually or together by means of site-directed mutagenesis of the pGL3.0-B4galnt1 and pGL3.0-miR-1931 promotor vectors. We co-transfected the wild-type promotors of B4galnt1 and miR-1931 or their mutated (MUT) counterparts along with NF-κB1 (p50) overexpression vectors into HEK293T cells. The outcomes revealed that NF-κB1 (p50) certain to sequences inside the B4galnt1 and miR-1931 promoters to boost their transcription (Fig. 3F and G). To additional affirm whether or not NF-κB1 (p50) immediately binds to the promotor areas of B4galnt1 and miR-1931 to affect their transcription, electrophoretic mobility shift assay (EMSA) was used to exhibit the bodily interactions between NF-κB1 (p50) and the promotor areas. The probes had been designed for NF-κB1 (p50) in keeping with its binding websites inside the promoter sequence, and the outcomes demonstrated that NF-κB1 (p50) did certainly bind to the respective binding websites. Tremendous-shift bands had been additionally clearly noticed after incubation with antibodies towards NF-κB1 (p50) (Fig. 3H and I).
NF-κB pathway enhancement of transcription of B4galnt1, the host gene of cir2047, and miR-1931 in macrophages. A–C Expression of cir2047, miR-1931, and miR-760 measured by qPCR in F4/80-positive cells at completely different time factors after polarization. D Immunofluorescence evaluation of NF-κB (p65) and NF-κB1 (p50) in F4/80-positive cells after M1 polarization. E Schematic of every NF-κB1 (p50)-binding website (pBS) within the promoter areas of B4galnt1 and miR-1931. F and G Impact of NF-κB1 (p50) on B4galnt1 and miR-1931 transcription by means of binding to their promotors was evaluated by luciferase reporter assays. H and I EMSA of bodily binding of NF-κB1 (p50) to the B4galnt1 and miR-1931 promotor areas; *p < 0.05; **p < 0.01; ns, not important
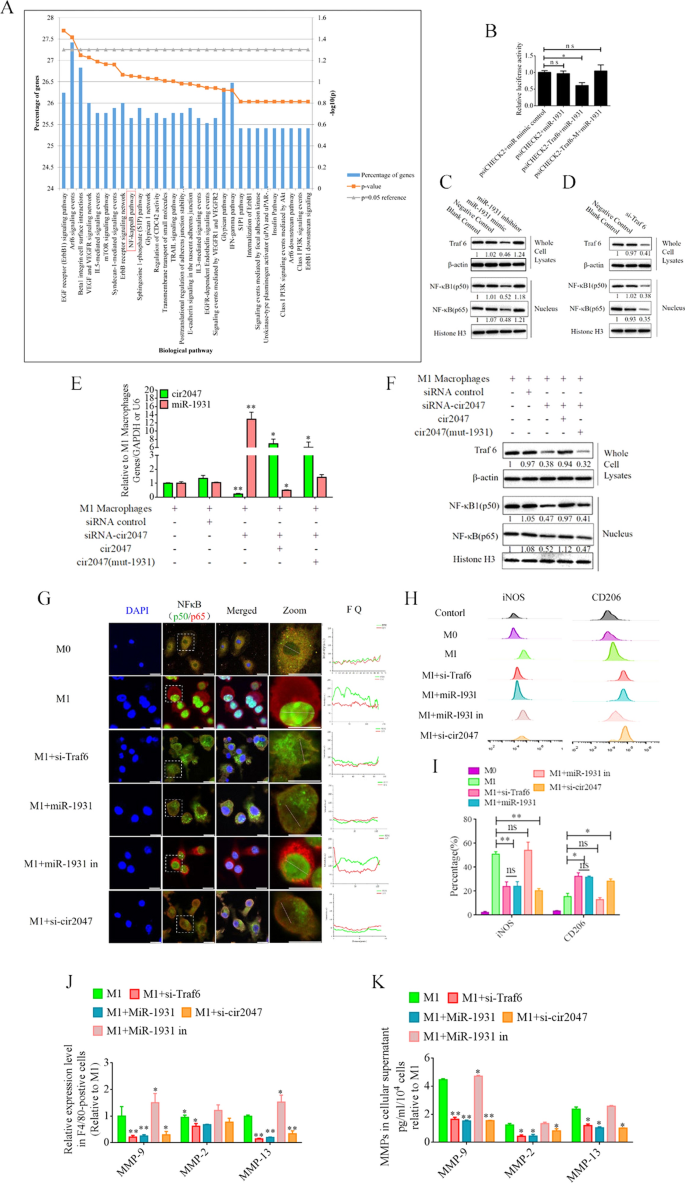
For example the position of cir2047 within the polarization of M1 macrophages, we analyzed the potential perform of miR-1931 targets utilizing the Useful Enrichment evaluation instrument (FunRich, http://www.funrich.org/). The highest 30 organic pathways of miR-1931 targets are proven in Fig. 4A. The expected goal of miR-1931, TRAF6, is a mediator of the NF-κB pathway and performs an necessary position in its activation. The small interfering RNA (siRNA) si-Traf6 was designed and utilized to M1 macrophages to exhibit that the degrees of NF-κB1 (p50) and NF-κB (p65) had been lowered dramatically following knockdown of Traf6 by siRNA (p < 0.05, Fig. 4C). MiRNAs are necessary regulators of varied organic processes that act by means of binding to the three’-untranslated areas (UTRs) of targets to modulate their expression. Bioinformatics algorithm prediction, luciferase reporter assays, and western blotting revealed that miR-1931 certain to the three’-UTR of Traf6 and controlled its expression (p < 0.05, Fig. 4B and D). MiR-1931 repressed TRAF6 expression within the cytoplasm, downregulating the degrees of p50 and p65 within the nucleus, whereas the alternative outcomes had been noticed after the addition of miR-1931 inhibitor (Fig. 4D). To additional look at the position of cir2047 in NF-κB pathway activation, particular siRNAs directed towards cir2047-wt and cir2047-mut had been transfected into M1 macrophages to research the RNA ranges of cir2047 and miR-1931 and the protein ranges of NF-κB1 (p50) and NF-κB (p65) within the nucleus. The information revealed that cir2047 immediately sponged miR-1931 to take care of activation of the NF-κB pathway (Fig. 4E and F). Fluorescein isothiocyanate (FITC)-labeled NF-κB1 (p50) and Cy5-labeled NF-κB (p65) had been used to judge localization. FITC and Cy5 fluorescence alerts had been initially noticed within the nuclei of M1 macrophages, however after remedy with si-Traf6, miR-1931, or si-cir2047, the FITC alerts had been co-localized with Cy5 within the cytoplasm. Fluorescence quantitation of arbitrary single cells revealed decrease FITC and Cy5 alerts within the teams with si-Traf6, miR-1931, or si-cir2047 remedy in contrast with the alerts within the group with M1 macrophages (Fig. 4G). Immunofluorescence confirmed that NF-κB1 (p50) and NF-κB (p65) had been successfully restricted to the cytoplasm after knockdown of Traf6 by siRNA, miR-1931, or si-cir2047. The expression charges of iNOS, a marker of M1 macrophages, and CD206, a marker of M2 phenotype macrophages, had been additional analyzed by circulate cytometry. The outcomes demonstrated that the share of CD206-positive cells within the teams handled with si-Traf6, miR-1931, or si-cir2047 had been considerably increased than that within the non-treatment group (Fig. 4H and I). Subsequent, the mRNA expression ranges of Mmp9, Mmp2, and Mmp13 had been detected in cell supernatants of M1 macrophages. Remedy with si-Traf6, miR-1931, and si-cir2047 successfully decreased the expression ranges of all three mRNAs (Fig. 4J and Okay). These information steered that TRAF6, a goal of miR-1931, had stabilized expression to take care of M1 polarization of macrophages by means of sponging by cir2047 to secrete MMPs in irritation reactions.
Cir2047 maintains activation of the NF-κB pathway and the secretion of MMPs by means of sponging miR-1931 in F4/80-positive cells. A The highest 30 organic pathways, together with the NF-κB pathway, generated by the expected targets of miR-1931. B The results of miR-1931 on TRAF6 expression had been validated utilizing luciferase reporter assays. The three’-UTR of Traf6 and sequences containing mutated variants of miRNA-binding websites (M) had been examined to find out their impact on miRNA expression. Outcomes are expressed as relative luciferase exercise and characterize the imply ± normal deviation of no less than three replicates. C Western blot exhibiting TRAF6 expression ranges in F4/80-positive cells at 48 h after transfection with particular siRNAs. The degrees of NF-κB (p65) and NF-κB1 (p50) in nuclei had been additionally decreased when Traf6 was knocked down. D Western blot exhibiting that miR-1931 decreased the expression of NF-κB (p65) and NF-κB1 (p50) within the nucleus by focusing on TRAF6 following remedy with an miRNA mimic. The relative density of bands is proven below every immunoblot after normalization to β-actin or histone ranges. Consultant blots from three unbiased experiments are proven. E qPCR exhibiting miR-1931 ranges in F4/80-positive cells after transgenesis. F Western blotting revealed expression of TRAF6 in cells and NF-κB (p65) and NF-κB1 (p50) in nuclei after transgenesis. The relative band density is proven below every immunoblot after normalization to β-actin or histone ranges. Consultant blots from three unbiased experiments are proven. G Immunofluorescence detected the activation of the NF-κB pathway in F4/80-positive cells after transgenesis. Fluorescence photographs used to judge the localization of FITC-labeled NF-κB1 (p50) and Cy5-labeled NF-κB (p65); FQ, fluorescence quantitation; a.u., arbitrary unit. H and I Move cytometry of M1 (iNOS) and M2 (CD206) macrophage markers in F4/80-positive cells after transgenesis and quantification of iNOS and CD206 expression ranges in F4/80-positive cells (n = 3). J mRNA ranges of Mmp9, Mmp2, and Mmp13 in F4/80-positive M1 polarized cells after transgenesis. Okay ELISA of MMP-9, MMP-2, and MMP-13 expression in mobile supernatants derived from F4/80-positive M1 polarized cells after transgenesis; *p < 0.05; **p < 0.01; ns, not important
EVs as a automobile to move si-Traf6, miR-1931, and si-cir2047 into macrophages
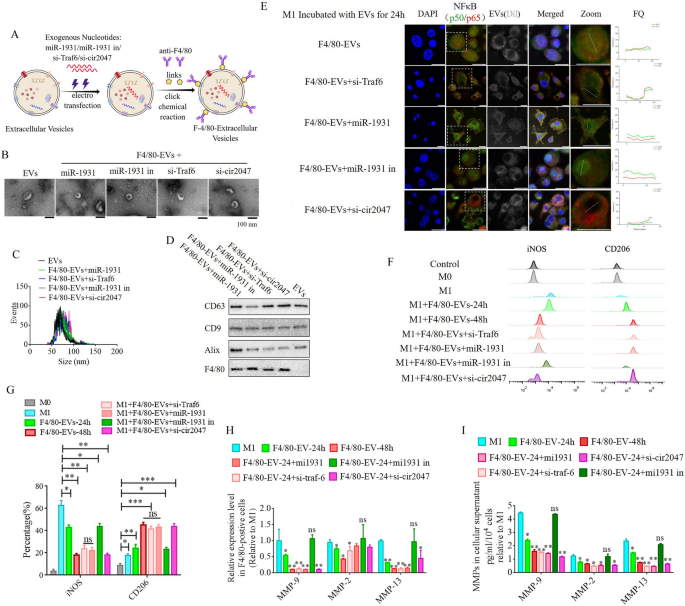
To additional analyze the roles of cir2047, TRAF6, and miR-1931 within the polarization of macrophages in vitro and in vivo, EVs from human ADSCs had been utilized as a automobile to move siRNAs towards cir2047, TRAF6 and miR-1931, or miRNA-1931 mimic, to stop M1 polarization in macrophages. First, the organic traits of human ADSCs had been recognized, together with particular markers and multi-differentiation potential. These outcomes demonstrated that CD29, CD90, and CD105 had been optimistic in human ADSCs, and that the ADSCs had efficiently differentiated into chondrocytes, adipocytes and osteoblasts (Extra file 1: Determine S1). Earlier experiences [13, 14] demonstrated that EVs derived from mesenchymal stem cells carry sure miRNAs and proteases to reverse the dominant phenotype from M1 to M2 in macrophages. On this examine, 1 × 108 particles/mL ADSC-EVs had been labeled with Dil after which incubated with M1 macrophages. The sign intensities of FITC-labeled NF-κB1 (p50) and Cy5-labeled NF-κB (p65) within the nucleus had been used to evaluate the activation of the NF-κB pathway after 24-h and 48-h remedy. Nonetheless, regardless of the sign being barely lowered at 24 h, the sign stage had fully diminished to the identical stage noticed in M0 macrophages after the 48-h remedy (p < 0.05, Extra file 1: Determine S2). Subsequent, EVs had been loaded with siRNAs towards cir2047, Traf6, and miR-1931 utilizing electroporation. To enhance the supply effectivity of those EVs in vivo, a rat monoclonal antibody towards F4/80 was certain to the floor of EVs utilizing click on chemistry (F4/80-EVs; Fig. 5A). First we in contrast the internalization efficiencies of F4/80-EVs and regular ADSC-EVs into recipient M1 macrophages. Dil-labeled EVs (1 × 108 particles/mL) incubated with M1 macrophages for twenty-four h revealed that the fluorescence stage was considerably increased in F4/80-EV-treated than in regular EV-treated M1 macrophages, implying that F4/80-EVs had been internalized extra effectively than regular EVs (p < 0.05, Extra file 1: Determine S3).
ADSC-secreted EVs as a automobile to move si-TRAF6, miR-1931, and si-cir2047 into macrophages in vitro. A Schematic illustration exhibiting that siRNAs had been loaded into ADSC-secreted EVs by electroporation. B–D Organic traits of those EVs, together with morphology, particulate measurement distribution, and EV marker proteins. E Fluorescent photographs of unloaded F4/80-EVs and si-RNA-loaded F4/80-EVs incubated with M1 macrophages for twenty-four h had been used to judge the localization of FITC-labeled NF-κB1 (p50) and Cy5-labeled NF-κB (p65). Fluorescence quantitation of arbitrary single cells revealed decrease FITC and Cy5 alerts within the group that obtained siRNA-loaded F4/80-EVs in contrast with the alerts within the group with unloaded F4/80-EVs; FQ, fluorescence quantitation; a.u., arbitrary unit. F and G Move cytometry of M1 (iNOS) and M2 (CD206) macrophage markers after remedy with varied F4/80-EVs and quantification of iNOS and CD206 expression ranges in varied F4/80-EV-treated macrophages (n = 3). H mRNA ranges of Mmp9, Mmp2, and Mmp13 in F4/80-positive M1 polarized cells after remedy with varied F4/80-EVs. I ELISA of MMP-9, MMP-2, and MMP-13 expression in mobile supernatant derived from F4/80-postive M1 polarized cells after remedy with varied F4/80-EVs; *p < 0.05; **p < 0.01; ***p < 0.001; ns, not important
Regular EVs and F4/80-EVs loaded with miR-1931 (F4/80-EVs + miR-1931), si-Traf6 (F4/80-EVs + si-Traf6), miR-1931 inhibitor (F4/80-EVs + miR-1931 in) or si-cir2047 (F4/80-EVs + si-cir2047) had been then analyzed for morphology, measurement, and attribute EV marker proteins. Transmission electron microscopy (TEM) confirmed that standard EVs and F4/80-EVs loaded with completely different siRNAs displayed a spherical form and had been intact, with no membrane harm (Fig. 5B). Nanoparticle monitoring evaluation revealed that the imply diameter sizes of regular EVs, F4/80-EVs + miR-1931, F4/80-EVs + si-Traf6, F4/80-EVs + miR-1931 in, and F4/80-EVs + si-cir2047 had been related (88.32 ± 9.47, 86.44 ± 10.54, 91.35 ± 7.61, 87.59 ± 8.74, and 90.18 ± 9.47 nm, respectively, Fig. 5C), and had been inside the measurement vary of identified exosomes (50–200 nm). Western blotting indicated that attribute EV marker proteins CD63, CD9, and Alix had been optimistic in all EVs, however F4/80 was not expressed in regular EVs (Fig. 5D).
Subsequent, M1 macrophages had been incubated with these EVs for twenty-four h, and immunofluorescence and circulate cytometry had been used to research NF-κB pathway activation and reversed macrophage polarization. The outcomes indicated that EVs loaded with these siRNAs may successfully cut back the sign depth of FITC-labeled NF-κB1 (p50) and Cy5-labeled NF-κB (p65) within the nucleus after 24 h, however excluded loaded miR-1931 mimic (Fig. 5E). Move cytometry information demonstrated that the share of CD206-positive cells within the group receiving F4/80-EVs loaded with siRNAs was considerably increased than that within the F4/80-EV group after 24 h, which was additionally noticed within the F4/80-EV group after 48 h (p < 0.05, Fig. 5F and G). Moreover, EVs loaded with si-Traf6, miR-1931, or si-cir2047 successfully decreased the mRNA expression of Mmp9, Mmp2, and Mmp13 in M1 macrophages after 24 h (p < 0.05, Fig. 5H), and protein expression of MMP-9, MMP-2, and MMP-13 was additionally declined in mobile supernatants (p < 0.05, Fig. 5I). The downtrend of MMP-9, MMP-2, and MMP-13 after 24 h within the teams handled with siRNA-loaded F4/80-EVs remained consistent with these handled with F4/80-EVs at 48 h. In beforehand examine reported that the exosomes derived from M2 macrophages inhibited tumor progress by reprogramming tumor-associated macrophages into M1-like macrophages [15], thus, the chances of the repolarization of the M2 to M1 phenotype was detected after remedy with these EVs on this analysis. To look at the consequences of those EVs in M2 macrophages following a 48-h incubation, circulate cytometry was used to detect potential reversed macrophage polarization. The outcomes indicated that the odds of iNOS-positive cells within the teams receiving siRNA-loaded F4/80-EVs weren’t considerably completely different from that in untreated M2 macrophages after 48 h (Extra file 1: Determine S4). These information implied that the F4/80-EV teams handled with siRNAs conferred the EVs with improved capability to transform the macrophage phenotype from M1 to M2 in vitro.
Focused supply of F4/80-EVs to macrophages in vivo and its therapeutic efficacy in PROM
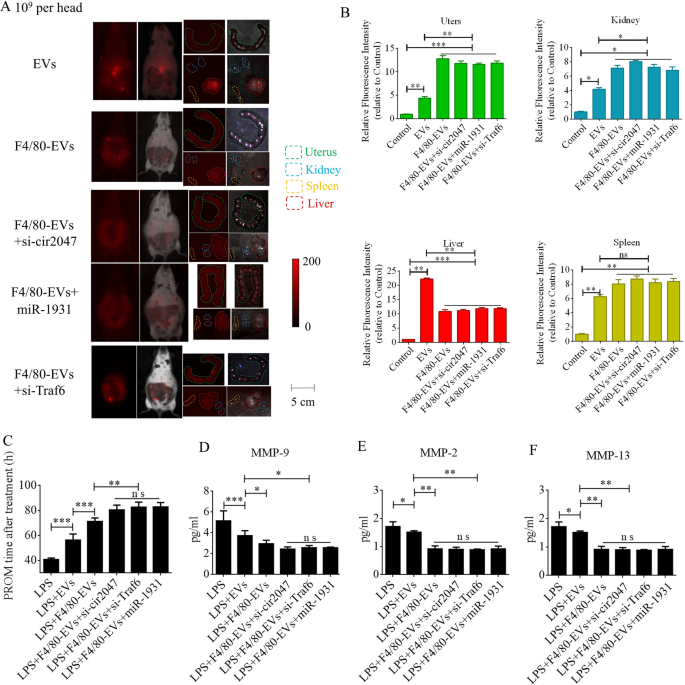
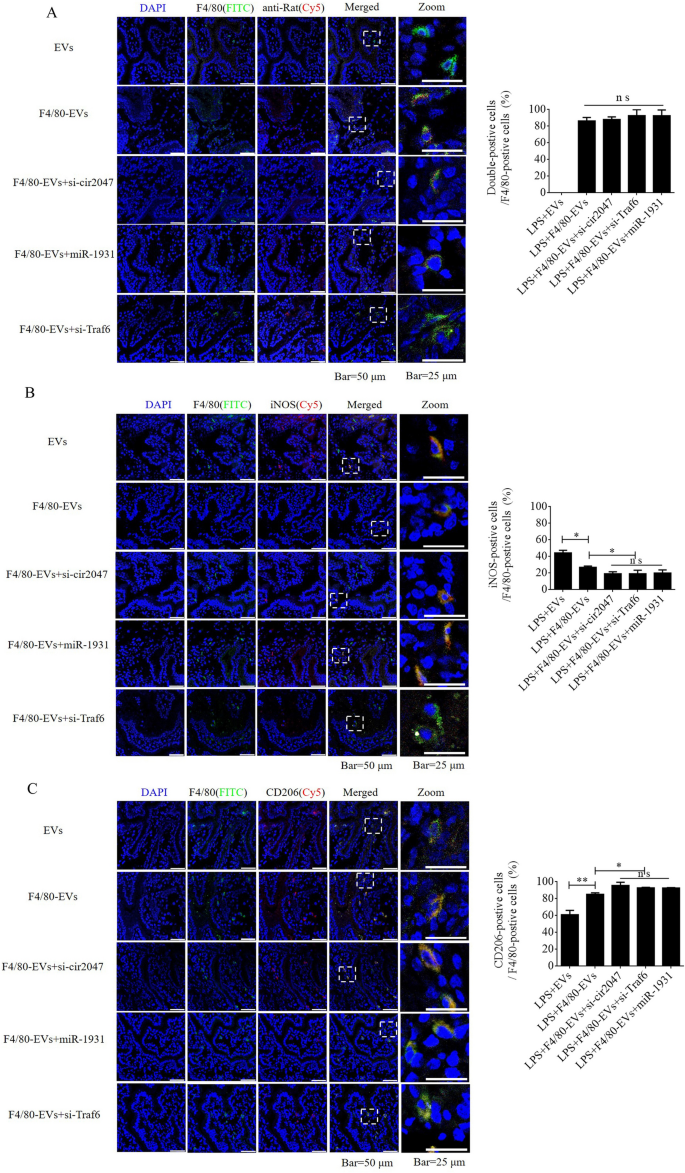
To additional confirm F4/80-mediated endocytosis of F4/80-EVs by macrophages, pregnant mice had been used to evaluate focused supply of EVs, F4/80-EVs, or siRNA-loaded F4/80-EVs (1 × 109 particles/mouse delivered by intrauterine injection) for the remedy of PROM. These EVs had been labeled with DiR, and their location was analyzed by entire physique fluorescence and organ fluorescence. Fluorescence alerts had been noticed with EV remedy, however not with the clean management. Most fluorescence alerts remained within the liver at 24 h after regular EV remedy, whereas the fluorescence alerts had unfold to the stomach of mice handled with F4/80-EVs and siRNA-loaded F4/80-EVs, and the uteri confirmed stronger fluorescence alerts than these in mice handled with EVs. In contrast with EVs, F4/80-EVs and siRNA-loaded F4/80-EVs principally gathered within the uterus, with some distribution within the kidneys, spleen, and liver of pregnant mice (Fig. 6A). The fluorescence depth of F4/80-EVs and siRNA-loaded F4/80-EVs within the uterus was considerably increased than that of EVs (Fig. 6B). Subsequent, we analyzed the timing of PROM incidence and located that it was prolonged after remedy with all EVs, however important modifications had been noticed within the teams receiving siRNA-loaded F4/80-EVs, together with si-TRAF6, si-cir2047, and miR-1931 mimic (Fig. 6C). Moreover, the expression ranges of MMP-9, MMP-2, and MMP-13 had been markedly lowered within the amniotic fluid of pregnant mice in any case EV therapies, with the obvious results noticed in mice handled with siRNA-loaded F4/80-EVs (Fig. 6D–F). To research the potential toxicity of those EVs in mice, ELISA was used to detect the inflammatory components TNF-α and IL-1β, the biochemical indicators of hepatotoxicity and nephrotoxicity, alanine transaminase (ALT), aspartate aminotransferase (AST), serum creatinine, and blood urea nitrogen. The information revealed that TNF-α, IL-1β, ALT, AST, serum creatinine, and blood urea nitrogen ranges had been considerably decreased in serum following remedy with these EVs in LPS-treated mice, which implied that there was no toxicity in vivo (Extra file 1: Determine S5). To confirm the focused supply of F4/80-EVs to macrophages in vivo, FITC-labeled rabbit polyclonal antibody towards F4/80 was used to find macrophages in uterine sections, and Cy5-labeled anti-rat antibody was used to find F4/80-targeted rat polyclonal antibody that was certain to the floor of EVs. Double-positive cells indicated that the macrophages contained these F4/80-EVs and confirmed that there have been no important modifications within the charge of double-positive cells within the teams handled with F4/80-EVs and F4/80-EVs loaded with si-Traf6, si-cir2047, or miR-1931 mimic (Fig. 7A). To research the consequences of EVs and these F4/80-EVs on macrophage polarization and their therapeutic efficacy in vivo, iNOS-positivity and CD206-positivity had been analyzed in F4/80-positive cells of uterine sections. The information revealed that the variety of iNOS-positive cells was markedly declined, and virtually 80% of F4/80-positive cells expressed CD206 after remedy with these F4/80-EVs. Moreover, the consequences had been stranger after remedy with F4/80-EVs loaded with si-Traf6, si-cir2047, or miR-1931 mimic (Fig. 7B and 7C). Taken collectively, our information indicated that cir2047 maintained the polarization of M1 macrophages and promoted MMP secretion by means of the miR-1931/TRAF6/NF-κB pathway to speed up PROM.
Therapeutic efficacies of various F4/80-EVs in PROM mice. A Consultant fluorescence imaging of varied F4/80-EVs (1 × 109 particles/mouse) in whole-body and organ tissues from PROM mice. B Relative fluorescence depth within the uterus, kidneys, liver, and spleen of PROM mice (n = 3). C Evaluation of PROM timing after varied F4/80-EV therapies within the PROM mouse mannequin (n = 5). D–F ELISA of MMP-9, MMP-2, and MMP-13 expression in amniotic fluid derived from regular management and PROM mannequin mice (n = 3); ns, not important; *p < 0.05; **p < 0.01; ***p < 0.001
Focused supply of F4/80-EVs into macrophages in vivo. A Location of F4/80-EVs in uterine sections from F4/80-EV-treated PROM mice utilizing the corresponding Cy5-labeled secondary antibody of F4/80. A rabbit polyclonal antibody towards F4/80 labeled with FITC was used to confirm macrophages. Double-positive cells indicated macrophages that carried F4/80-EVs (n = 3). B and C Consultant immunofluorescence photographs of iNOS- and CD206-positive cells within the uteri of PROM mice handled with varied F4/80-EVs and quantification of iNOS- and CD206-positive cells in uterine tissues following these therapies (n = 3); *p < 0.05; **p < 0.01; ***p < 0.001; ns, not important