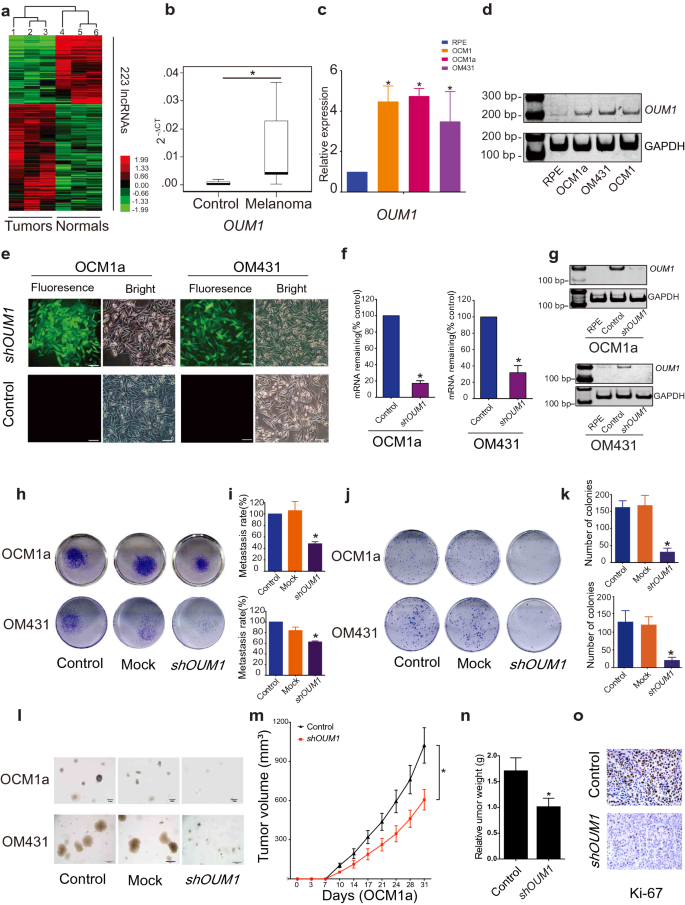
OUM1 is overexpressed in melanoma tissues and UM cells, and the knockdown of OUM1 can inhibit cell proliferation, invasion and xenograft development
To analyze lncRNA expression in melanoma, a microarray containing probes focusing on 12,784 lncRNAs was used, and greater than 200 lncRNAs have been discovered to be considerably differentially expressed (P < 0.05; fold change > 2) in three pairs of eyelid melanoma and regular tissue. Hierarchical clustering confirmed systematic variations in lncRNA expression between melanoma and regular tissues (Fig. 1a). Info from the Nationwide Heart for Biotechnology Info (NCBI) for LOC100505912 (Further file 1: Fig. S1a), which was considerably upregulated in melanoma tissues (Fig. 1b) and UM cells (Fig. 1c, d), is offered. As detailed in UCSC and NCBI databases, LOC100505912 has a size of 898 bp, incorporates 4 exons and is situated at 4p15.2 (Further file 1: Fig. S1b, yellow field). By speedy amplification of cDNA ends (RACE), we recognized a novel 1,464 bp transcript spanning 7 exons in UM cells (Further file 1: Fig. S1b, blue field). Utilizing the GENCODE annotation of the human genome, we then confirmed the absence of coding proof for this novel transcript. Collectively, these knowledge present that this novel isoform of LOC100505912 is a noncoding transcript in UM and a possible new oncoRNA, and we due to this fact named it Oncotarget in UM Formation-Transcript 1 (OUM1).
OUM1 overexpressed in melanoma tissues and UM cells and OUM1 knockdown inhibited cell proliferation and invasion. a Hierarchical clustering evaluation of 223 lncRNAs that have been differentially expressed between melanoma samples and nontumor samples. b The lncRNA OUM1 was considerably overexpressed in melanoma tissues in contrast with regular tissues. c OUM1 confirmed greater expression within the melanoma cell strains OCM1, OCM1a and OM431 than in RPE cells (unfavourable management), as measured by qRT-PCR. d OUM1 expression was measured by RT-PCR in numerous UM and regular cells. Triplicate assays have been carried out and the relative stage of OUM1 was normalized to GAPDH (*P < 0.05). e OUM1 was knocked down by one shRNA. EGFP was utilized to trace OUM1 shRNA expression. f, g OUM1 was downregulated in UM cells, as proven by qRT-PCR and RT-PCR. h Photos of the Transwell assays. i Migrated cells that have been fastened with 5% paraformaldehyde, stained with 0.1% crystal violet and washed with 33% acetic acid have been quantified. The absorbance of the collected liquid was measured at 570 nm with a microplate reader. j Photos of colony formation assays. okay Colonies have been fastened, stained, washed and measured with a microplate reader at 570 nm. l Photos of colonies in tender agar. Few small colonies have been noticed within the OUM1 knockdown group. m Nu/Nu nude mice have been injected with OCM1a or shOUM1-OCM1a cells. Tumor quantity was measured twice per week, each 3 or 4 days. The xenografts grew slowly in mice injected with shOUM1-OCM1a cells in comparison with these injected with management cells (n = 6 mice per group). n After 31 days, the mice have been sacrificed, and the tumors have been eliminated and analyzed. The tumors from mice injected with shOUM1-OCM1a cells weighted much less. o Ki-67 expression was evaluated by IHC staining in mouse tumor tissues. Scale bar: 20 μm. Mock: empty pGIPZ vector; *P < 0.05 in contrast with the management
Subsequently, three siRNAs have been designed for the knockdown of OUM1 expression (Further file 1: Fig. S1b-e). The siRNA-mediated knockdown of OUM1 considerably decreased cell viability and cell migration (Further file 1: Fig. S1f, g). As well as, we discovered excessive effectivity S2 splice websites in exon 7 of OUM1, which have been chosen to assemble the pGIPZ OUM1 shRNA plasmid. The pGIPZ shRNA vector harboring EGFP was packaged into lentiviruses and transduced into human OCM1a and OM431 cells. We noticed inexperienced fluorescence within the transduced cells (Fig. 1e). We chosen cell clones utilizing puromycin and detected OUM1 expression; OUM1 expression was knocked down in shOUM1-OCM1a and shOUM1-OM431 cells (Fig. 1f, g). The 24-well transwell experiment indicated that the metastasis fee was decreased by 50% in shOUM1-OCM1a cells and by roughly 40% in shOUM1-OM431 cells, and each of those charges have been markedly decrease than these of the untreated OCM1a and OM431 cells (Fig. 1h, i). Subsequently, decreased colony numbers have been noticed in shOUM1-OCM1a cells and shOUM1-OM431 cells by the colony formation assay (Fig. 1j, okay). As well as, the tender agar assay displayed fewer and smaller colonies in shOUM1-OCM1a cells and shOUM1-OM431 cells (Fig. 1l). These in vitro knowledge indicated that OUM1 promoted UM development. In vivo experiments utilizing nude mice have been subsequently carried out. Untreated OCM1a and the shOUM1-OCM1a cells have been individually injected into nude mice (every group consisted of six mice). The tumor quantity was measured as soon as each 3–4 days (twice per week). The tumors grew extra slowly in mice injected with shOUM1-OCM1a cells than in mice injected with untreated OCM1a cells (Fig. 1m). After 31 days, the mice have been sacrificed, and the tumors have been eliminated and analyzed. The tumor weights have been decrease within the shOUM1-OCM1a group (Fig. 1n). As noticed by IHC staining, much less Ki-67 expression was present in mouse tumor tissue of the shOUM1-OCM1a group on the finish of the experiments (Fig. 1o). These knowledge indicated that the proliferation and metastasis charges have been impaired after OUM1 knockdown in vivo and in vitro. OUM1 would possibly function a brand new oncoRNA in UM.
OUM1 contributes to tumor development by means of its downstream goal PTPRZ1
To analyze the function of OUM1 in UM, a genome-wide cDNA array was employed to find out elements after OUM1 knockdown. In contrast with the expression in untreated OCM1a cells, OUM1 knockdown downregulated and upregulated the expression of 86 and 63 genes, respectively (Further file 1: Desk S1). The molecular perform evaluation revealed that nucleotide binding, calcium ion binding, protein homodimerization exercise, GTPase exercise, integrin binding, development issue exercise, and extracellular matrix binding have been considerably modified after OUM1 knockdown. Nucleotide binding accounted for the biggest proportion, which was roughly 32.1% (Further file 1: Fig. S2). Bioformational evaluation additional verified that nucleotide-binding exercise was related to transmembrane receptor protein phosphatase, which was carefully associated to PTP exercise (Further file 1: Fig. S3). Among the many differentially expressed genes after OUM1 knockdown, just one gene, PTPRZ1, was related to PTPs. The expression of PTPRZ1 was considerably downregulated by roughly 3.9-fold (Further file 1: Desk S1). Different enzyme actions related to genes weren’t detected; that’s, PTPRZ1 would possibly act as a goal of OUM1 and would possibly perform as an oncogene in UM cells.
We additionally demonstrated that PTPRZ1 expression was decreased at each the mRNA stage and protein ranges after OUM1 knockdown (Fig. 2a–c), whereas ample PTPRZ1 expression was detected at each the mRNA stage and protein stage in untreated UM cells (Fig. 2d–g). To confirm the medical significance of PTPRZ1, we examined whether or not PTPRZ1 expression is correlated with OUM1 expression in tumor tissues. PTPRZ1 was abundantly expressed in tumor tissues (Fig. 2h) in accordance with the expression of OUM1. Thus, PTPRZ1 could be a goal of OUM1 and performance as an oncoRNA in UM cells. We then knocked down PTPRZ1 utilizing two completely different siRNAs (siPTPRZ1-1 and siPTPRZ1-2) designed to silence PTPRZ1 expression in OCM1a and OM431 cells. As anticipated, PTPRZ1 was efficiently silenced by siPTPRZ1-1 and siPTPRZ1-2 (Further file 1: Fig. S4a, b). Intriguingly, OUM1 expression was not considerably modified in UM cells after the silencing of PTPRZ1 (Further file 1: Fig. S4c). To exclude off-target results, we additionally investigated two downregulated genes, FXYD3 and ST8SIA1. Nevertheless, there have been no vital modifications in FXYD3 or ST8SIA1 expression have been present in shOUM1-OCM1a and shOUM1-OM431 cells or in melanoma samples (Further file 1: Fig. S4d-g). These knowledge additional verify that PTPRZ1 is the downstream regulatory goal of OUM1.
OUM1 contributes to tumor development by means of its downstream goal PTPRZ1. a, b The mRNA expression of PTPRZ1 was upregulated in untreated UM cells however downregulated after OUM1 knockdown, as detected utilizing qRT-PCR and native PAGE in OCM1a, OM431, shOUM1-OCM1a and shOUM1-OM431 cells. c The protein expression of PTPRZ1 decreased, as decided by western blot. d, e PTPRZ1 expression was greater in a panel of UM cell strains (OCM1, OCM1a, OM431, VUP, 92–1 and C918) than in management RPE cells as measured by qRT-PCR and RT-PCR. f, g PTPRZ1 expression was greater in a panel of UM cell strains than in regular RPE cells as proven by western blot. h In melanoma tissues, PTPRZ1 expression elevated considerably, as detected by qRT-PCR. *P < 0.05 in contrast with the management. i, j Cell proliferation was considerably decreased in OCM1a and OM431 cells after PTPRZ1 silencing. okay Photos of metastatic PTPRZ1-silenced tumor cells. The migration assay was performed 48 h after transfection with siPTPRZ1 or management siRNA. l, m Quantitative evaluation of metastatic tumor cells. The migratory potential of siPTPRZ1-treated OCM1a and OM431 tumor cells was considerably decreased. n IHC staining confirmed that PTPRZ1 protein expression was decreased in mouse tumors shaped by shOUM1-OCM1a cells. o PTPRZ1 expression was decreased in mouse tumors shaped by shOUM1-OCM1a cells, as proven by qRT-PCR. *P < 0.05 in contrast with the management
Subsequently, the regulatory function of PTPRZ1 in tumorigenesis was elucidated. By means of MTT assays, we noticed a decreased proliferation fee in OCM1a and OM431 cells after the silencing of PTPRZ1 expression (Fig. 2i, j). Classical transwell assays confirmed a considerably decreased metastasis fee, which was in line with the outcomes after OUM1 knockdown (Fig. 2okay–m). We additionally detected PTPRZ1 expression in tumor tissues from Nu/Nu nude mice injected with OCM1a or shOUM1-OCM1a cells. In a nude mouse xenograft mannequin shaped by shOUM1-OCM1a cells, decreased PTPRZ1 expression was detected by immunohistochemistry (IHC) staining and quantitative reverse transcription-polymerase chain response (qRT-PCR) (Fig. 2n, o).
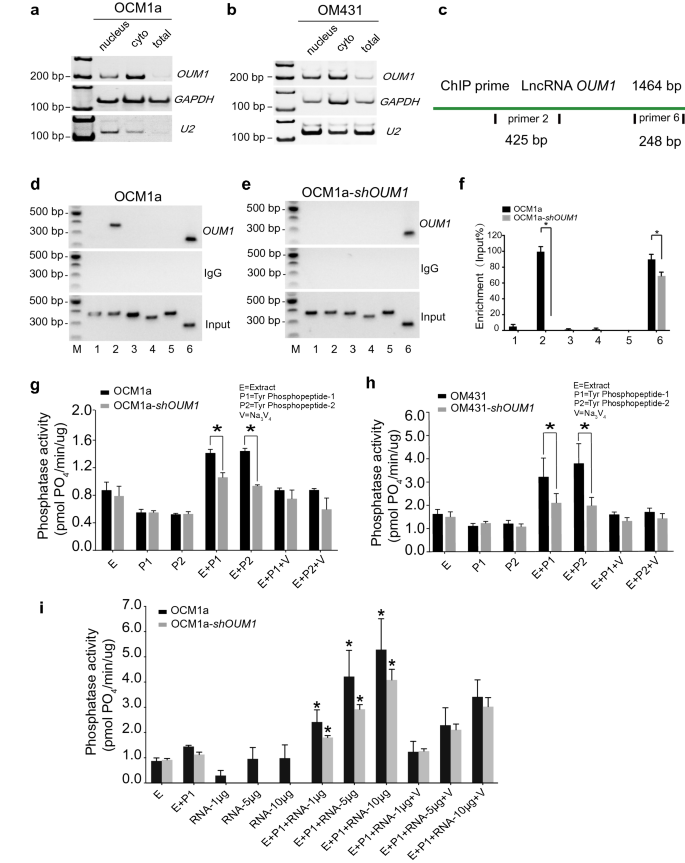
OUM1 is principally localized within the cytoplasm and straight binds to the PTPRZ1 protein to boost PTP exercise
To realize perception into the underlying mechanism by means of which OUM1 regulates PTPRZ1 expression in UM, we examined the subcellular location of OUM1. By isolating nuclear, cytoplasmic and whole RNA, OUM1 was demonstrated to be primarily localized within the cytoplasm in OCM1a and OM431 cells (Fig. 3a, b). U2 snRNA was used as a constructive reference [36].
OUM1 primarily locates in cytoplasm and straight binds to PTPRZ1 protein to boost PTP exercise. a, b OUM1 primarily situated within the cytoplasm in OCM1a and OM431 cells. U2 RNA: constructive management for nuclear RNA. c Schematic of the areas chargeable for binding between OUM1 and PTPRZ1. Websites 2 and 6: completely different detection areas for RNA ChIP. d, e The interplay of OUM1 and PTPRZ1 was detected. After OUM1 knockdown, the binding between PTPRZ1 and OUM1 was abolished on the primer 2 area and decreased on the primer 6 area in OCM1a-shOUM1 cells. Enter: whole RNA reverse transcribed and amplified with out incubation with the PTPRZ1 antibody. IgG: unfavourable management. f Grey stage evaluation performed to quantify the enrichment of OUM1 on PTPRZ1. g, h PTP exercise was detected in OCM1a and OM431 cells. The quantity of phosphate signifies phosphatase exercise. The quantity of phosphate decreased from 1.4 pmol PO4/min/μg and 1.5 pmol PO4/min/μg to 1.0 pmol PO4/min/μg and 0.9 pmol PO4/min/μg when P1 and P2 have been added, respectively, after OUM1 knockdown in OCM1a cells. In OM431 cells, these values decreased from 3.2 pmol PO4/min/μg and three.7 pmol PO4/min/μg to 2.1 pmol PO4/min/μg and a pair of.0 pmol PO4/min/μg when P1 and P2 have been added, respectively, after OUM1 knockdown. i Phosphate content material elevated considerably after synthesized OUM1 RNA was added to the PTP response system at growing concentrations of 1 μg, 5 μg and 10 μg; at these concentrations, the phosphate content material was roughly 2.6 pmol PO4/min/μg, 4.2 pmol PO4/min/μg, and 5.2 pmol PO4/min/μg, respectively, in OCM1a cells and 1.8 pmol PO4/min/μg, 2.9 pmol PO4/min/μg, and 4.0 pmol PO4/min/μg, respectively, in shOUM1-OCM1a cells. E Extract, protein extracted from OCM1a, OM431, shOUM1-OCM1a or shOUM1-OM431 cells. P1 and P2 point out two chemically synthesized phosphopeptides, Tyr phosphopeptides 1 (END(pY)INASL) and a pair of (DADE(pY)LIPQQG). SOV (V): Na3VO4, a PTP inhibitor that inhibits PTP exercise and is used as constructive management. *P < 0.05
Most research have targeted on lncRNAs localized within the nucleus and on their perform by means of interactions with gene promoters or competitors with a wide range of histones; fewer research have investigated cytoplasmic lncRNAs [37]. Subsequently, the mechanism of the lncRNA OUM1 was additional explored. The RNA-chromatin immunoprecipitation (RNA-ChIP) assay confirmed that OUM1 features primarily based on binding to the PTPRZ1 protein. Two binding websites for OUM1 on PTPRZ1 have been detected with primer 2 and primer 6 (Fig. 3c). In shOUM1-OCM1a cells, the primer 2 binding website between OUM1 and PTPRZ1 was virtually utterly deleted, whereas that of primer 6 was impaired (Fig. 3d–f). This discovering demonstrates that OUM1 can perform by straight binding to the PTPRZ1 protein within the cytoplasm.
We subsequently detected PTP exercise primarily based on the phosphate content material of OCM1a and OM431 cells (Fig. 3g, h). Sodium orthovanadate (SOV, V) is a PTP inhibitor that has been employed as a unfavourable management. In our examine, PTP exercise was activated solely within the presence of each mobile protein extract (E) and substrate (two chemically synthesized phosphopeptides, P1: END(pY)INASL and P2: DADE(pY)LIPQQG). When the reactions contained solely E, P1 or P2, the quantity of phosphate was virtually unchanged. After each E and P1 or P2 have been added, the enzymes have been considerably activated, and extra phosphate was produced in OCM1a and OM431 cells, which overexpress OUM1. Nevertheless, much less phosphate was produced after OUM1 knockdown. As well as, after the addition of various concentrations of OUM1 RNA to the PTP response system, which was amplified, confirmed and purified, we found that the phosphate content material was considerably elevated in a dose-dependent method (Fig. 3i). Nevertheless, when OUM1 RNA alone was added, the quantity of phosphate shaped was as little as that obtained when solely E was added. A earlier examine confirmed that OUM1 may straight bind to the PTPRZ1 protein. We discovered that the novel cytoplasmic lncRNA OUM1 acts as a catalyst by binding to PTPRZ1 to boost PTP exercise in UM cells and that the inhibition of OUM1 may inhibit the proliferation and metastasis of UM in vivo and in vitro by interrupting protein tyrosine phosphorylation within the UM microenvironment. Given the sturdy upregulation of each OUM1 and PTPRZ1 in human malignant melanoma tissues and the discovering that OUM1 knockdown inhibits the expression of OUM1 and subsequently decreases PTPRZ1 exercise, focusing on OUM1 and the downstream PTPRZ1 could also be a promising technique to inhibit the proliferation and metastasis of UM.
SOV has proven promising antineoplastic exercise in a number of human cancers [38, 39]. Nevertheless, the consequences of SOV on UM are comparatively unknown. On this examine, we detected the organic impact of UM cells after incubation with completely different concentrations of SOV. A MTT assay decided that the cell viability of OCM1a, shOUM1-OCM1a, OM431, shOUM1-OM431, MUM2b, OCM1, VUP and SP6.5 cells was suppressed in a dose-dependent method. Incubation with growing concentrations of SOV (10 μM, 25 μM, 50 μM and 100 μM) for twenty-four h, 48 h, 72 h and 96 h considerably suppressed the viability of UM cells (Further file 1: Fig. S5a-h); that’s, the survival fee was considerably decrease in shOUM1-OCM1a cells (Further file 1: Fig. S5e, f) and shOUM1-OM431 cells (Further file 1: Fig. S5g, h) than within the management teams. These knowledge recommend that lncRNA OUM1 knockdown may improve the antitumor exercise of SOV and enhance drug tolerance. This discovering signifies that the appliance of chemical medicine corresponding to cisplatin when OUM1 and PTPRZ1 are selectively knocked down and the OUM1/PTPRZ1 pathway is inhibited may drastically enhance drug tolerance and enhance antitumor efficacy.
Characterization of NPs
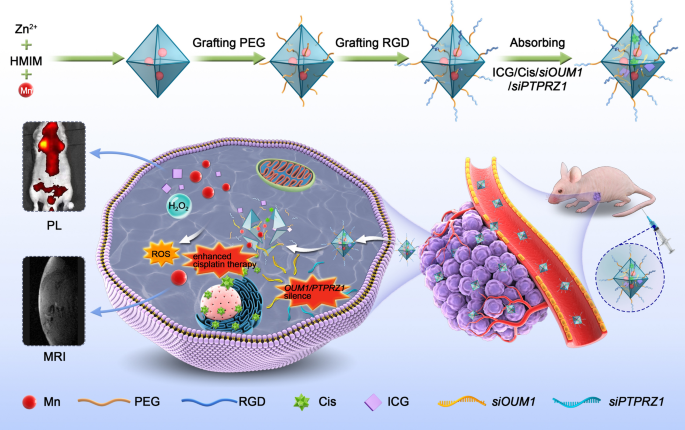
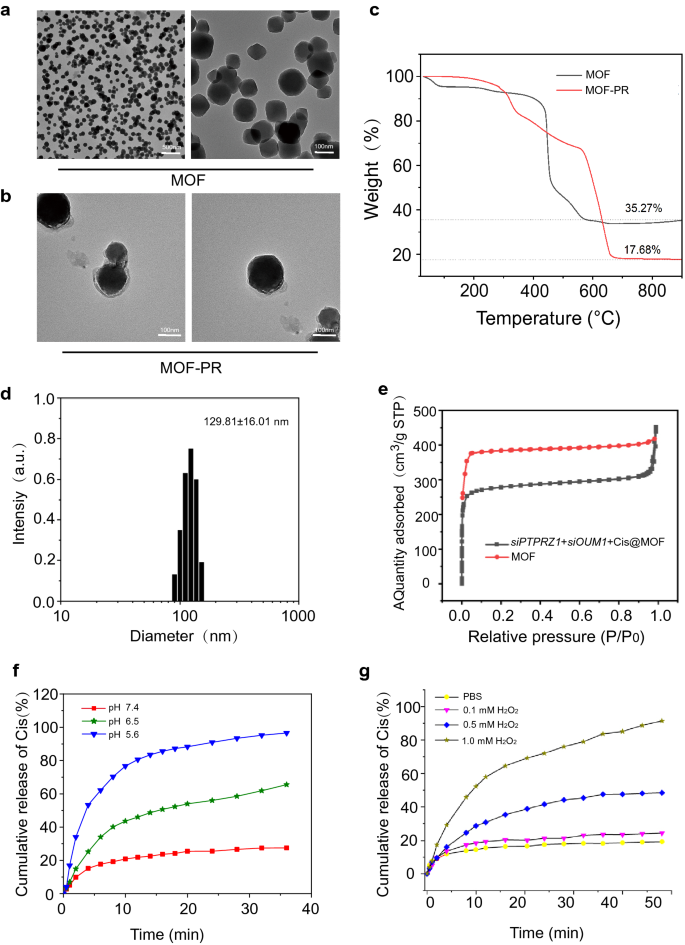
At current, RNA interference-mediated remedy has suffered from an absence of efficient oligonucleotide-delivery methods. Conventional intravenous chemotherapy lacks specificity, and chemotherapy medicine additionally produce poisonous and hostile unwanted effects on regular tissues. To deal with the shortage of efficient oligonucleotide-delivery methods, drug supply NPs primarily based on Mn@MOF have been designed to beat the restrictions of free therapeutics and to navigate organic boundaries, as illustrated in Fig. 4. The MOF and MOF building strategies are described within the experimental part. The TEM photographs in Fig. 5a and Fig. 5b revealed the uniform polyhedron morphology of MOF and MOF-RGD-PEGTK (MOF-PR) inside nanoscale dimensions. The MOF floor grew to become rougher after PEG and RGD ornament, and the diameter of the NPs was elevated. The MOF and MOF-PR confirmed ca. 62.64% and 81.98% mass loss, respectively, in contrast with the dad or mum MOF (Fig. 5c), which signifies that roughly 19.34% RGD and PEG have been absorbed into the MOF NPs. As decided by DLS, the hydrodynamic diameter of MOF-PR was 129.81 ± 16.01 nm (Fig. 5d). As illustrated in Fig. 5e, each exhibited a typical sort I N2 absorption − desorption isotherm, which is taken into account one of many main traits of microporous supplies. The BET floor space of the MOF was 1176 m2 g−1, whereas this worth decreased to 563 m2 g−1 for siPTPRZ1 + siOUM1 + Cis@MOF. Moreover, the corresponding pore volumes of siPTPRZ1 + siOUM1 + Cis@MOF and pure MOF nanostructures have been 0.19 cm3 g−1 and 0.79 cm3 g−1, respectively. The sharp discount within the BET floor space and pore quantity demonstrated ample drug molecules contained in the MOF NPs. On this examine, Zn2+ was a necessary component in establishing the MOF and the construction ready from zinc ion and 2-methylimidazolate is secure below almost impartial aqueous setting however might be decomposed in acidic situations [40]. This enabled the pH-responsive drug supply characterization of the MOF. To discover the pH-responsiveness of the nanoparticles, the discharge profile of cisplatin from Cis@MOF-PR below pH 5.6, 6.5 and seven.4 was carried out. As proven in Fig. 5f, a 40-min sustained launch profile of cisplatin from Cis@MOF-PR NPs was noticed. The quantity of drug launched by Cis@MOF-PR NPs was considerably greater below answer with decrease pH. The drug launched by the NPs was acidic sensitivity. Furthermore, on this examine, MOF was coated with TK linkers, which is ROS- responsive. When simulated with ROS, the ROS-responsive TK linkers would break and medicines loaded within the pores of MOF would launch [41]. To additional examine the ROS-responsiveness of the nanoparticles, the discharge profile of cisplatin from Cis@MOF-PR was carried out in phosphate buffered saline (PBS), H2O2 and acidic H2O2. As illustrated in Fig. 5g, a 60-min sustained launch profile of cisplatin from Cis@MOF-PR NPs was noticed. Roughly 90% of cisplatin was launched from the nanovehicles inside 48 min in acidic H2O2 with 1 mM H2O2, whereas in H2O2 and PBS, the quantity was diminished to 54.0% and 29.3%, respectively, over the identical period. The H2O2, in addition to acidity had a constructive and vital influence on the discharge fee of cisplatin from Cis@MOF-PR NPs. These evidences demonstrated that the options of the drug launched by the nanocarrier have been ROS and acidic sensitivity.
Synthesis and characterization of NPs. a TEM photographs of MOF. b TEM photographs of MOF-PR. c Thermogravimetric evaluation of MOF and MOF-PR. d The NP dimension distribution of MOF-PR. e Nitrogen adsorption of MOF and siOUM1 + siPTPRZ1 + Cis@MOF. f Cisplatin launch from Cis@MOF-PR in options with completely different pH values. g Cisplatin launch from Cis@MOF-PR below acidic and H2O2 situations
NPs might be taken up into UM cells to carry out the cell-killing impact
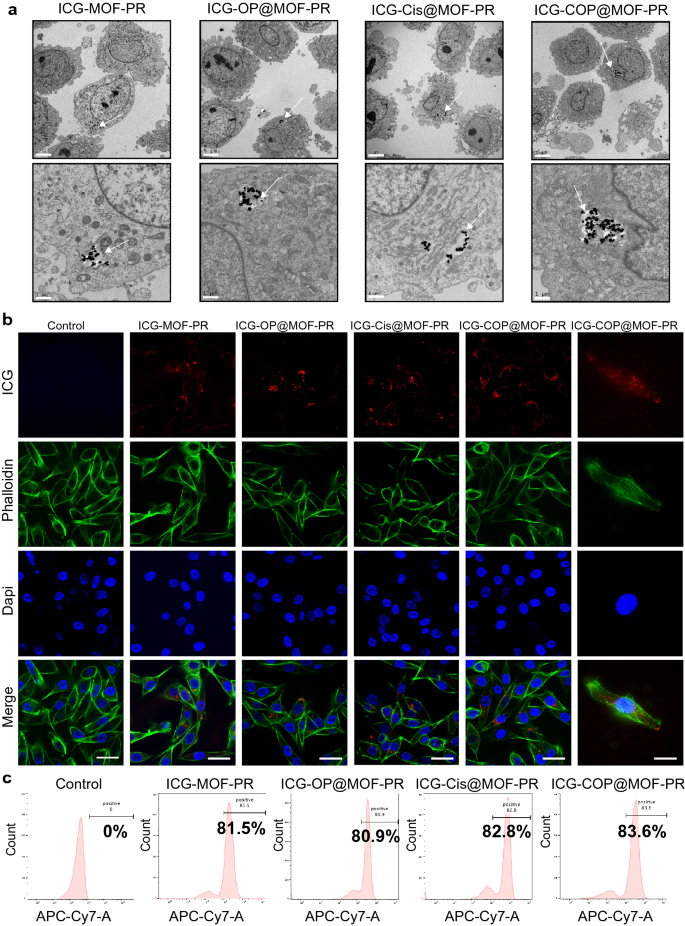
The mobile uptake and intracellular distribution of NPs are crucial for his or her organic actions. To analyze the mobile uptake of NPs, OCM1a cells have been incubated with ICG-MOF-PR, ICG-OP@MOF-PR (ICG-siOUM1 + siPTPRZ1@MOF-PR), ICG-Cis@MOF-PR and ICG-COP@MOF-PR in a single day at 37 ℃ after which the uptake of NPs was the investigated by TEM, which confirmed the incorporation of NPs into OCM1a cells (Fig. 6a). ICG in NPs serves as a fluorescence probe permitting for detection by CLSM and IVIS. OCM1a cells have been then incubated with NPs in a single day at 37 ℃, and the fluorescence depth of ICG was then analyzed by CLSM. The outcomes additional confirmed the uptake of fluorescent NPs into OCM1a cells (Fig. 6b). The ICG fluorescence depth of OCM1a cell-incubated NPs was additionally noticed by way of a move cytometer (Fig. 6c).
Mobile uptake of NPs. a TEM photographs point out the intracellular distribution of NPs in OCM1a. Arrows: NPs uptaken into the cells. The size bars within the first row and the second row are 5 μm and 1 μm, respectively. b From as much as down, the CLSM photographs present ICG labeled NPs (crimson), cytoskeleton stained with FITC fluorescence-phalloidin (inexperienced), and cell nuclei stained with DAPI (blue) in OCM1a. The size bars within the left 5 columns and the correct 1 column are 50 μm and 20 μm, respectively. c Mobile uptake of NPs evaluated by move cytometer. Experiments have been repeated for 3 unbiased instances
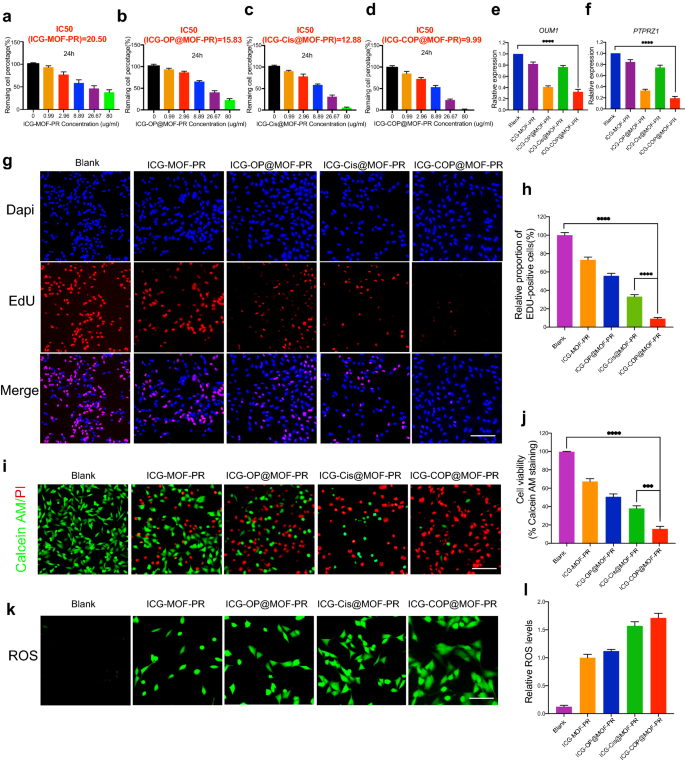
Additional research have been then performed to judge the antitumor capacities of the NPs. First, CCK8 was employed to check cell viability. The outcomes proven in Fig. 7a–d point out dose-dependent cell viability. The IC50 values of ICG-MOF-PR, ICG-OP@MOF-PR, ICG-Cis@MOF-PR and ICG-COP@MOF-PR on OCM1a cells have been 20.50, 15.83, 12.88 and 9.99 μg mL−1, respectively. The PCR outcomes demonstrated that OUM1 and PTPRZ1 expression in OCM1a cells was considerably knocked down by ICG-OP@MOF-PR and ICG-COP@MOF-PR (Fig. 7e, f). EdU staining was then carried out utilizing OCM1a cells incubated with NPs for twenty-four h. The outcomes revealed that the cell proliferation inhibition impact of ICG-COP@MOF-PR was drastically improved in contrast with that of ICG-Cis@MOF-PR (Fig. 7g, h). The CCK8 and EdU outcomes have been additionally echoed by stay and useless staining of the cells after varied remedies (Fig. 7i, j). Once more, ICG-COP@MOF-PR exhibited the best cell killing impact, whereas partial cell loss of life was noticed with ICG-OP@MOF-PR and ICG-Cis@MOF-PR. The MOF system constructed in our examine degraded within the mildly acidic tumor microenvironment and launched medicine loaded within the micropores and Mn2+, which induced a Fenton-like response and produced cytotoxic ROS to exert a cell killing impact. We then noticed the ROS manufacturing induced by ICG-MOF-PR, ICG-OP@MOF-PR, ICG-Cis@MOF-PR and ICG-COP@MOF-PR. As proven in Fig. 7okay, l, ROS have been detected in OCM1a cells handled with these MOF NPs, which indicated the excessive CDT efficacy of this NP system. Taken collectively, the outcomes point out that ICG-COP@MOF-PR exhibited the most effective cell killing impact by means of a mixture of ROS technology, selective siRNA knockdown and enhanced cisplatin therapy.
Organic exercise of NPs. a–d Dose-dependent cell viability of OCM1a and IC50 values of NPs at 24 h. e, f Lnc OUM1 and PTPRZ1 expression in OCM1a after handled with NPs for 48 h. g, h EDU staining of OCM1a after handled with completely different NPs for twenty-four h. Scale bars, 200 μm. i, j Cell survival/loss of life by stay/useless staining (Calcein AM: stay cells, PI: useless cells). Scale bars, 200 μm. okay, l ROS manufacturing of OCM1a after handled with completely different NPs for twenty-four h. Scale bars, 100 μm. All knowledge have been obtained from no less than three unbiased experiments (n ≥ 3), *** P < 0.001. **** P < 0.0001
In vivo evaluation of the pharmacokinetics, focusing on impact and dual-modal imaging capability of intravenously injected NPs
Furthermore, the pharmacokinetics of intravenously injected NPs have been assessed to disclose the in vivo behaviors. At concentrations of 0, 5, 10, 15, and 20 mg kg−1, NPs have been injected intravenously into Kunming mice. Initially, a number of main organs, together with the center, liver, lung, spleen, and kidney, have been subjected to histological evaluation after therapy with the indicated NP concentrations for 30 days. Hematoxylin and eosin staining of the abovementioned main organs in numerous teams confirmed no marked tissue abnormalities (Further file 1: Fig. S6a). As well as, there was no vital distinction in mouse physique weights among the many completely different teams (Further file 1: Fig. S6b), additional evidencing the wonderful biocompatibility of NPs.
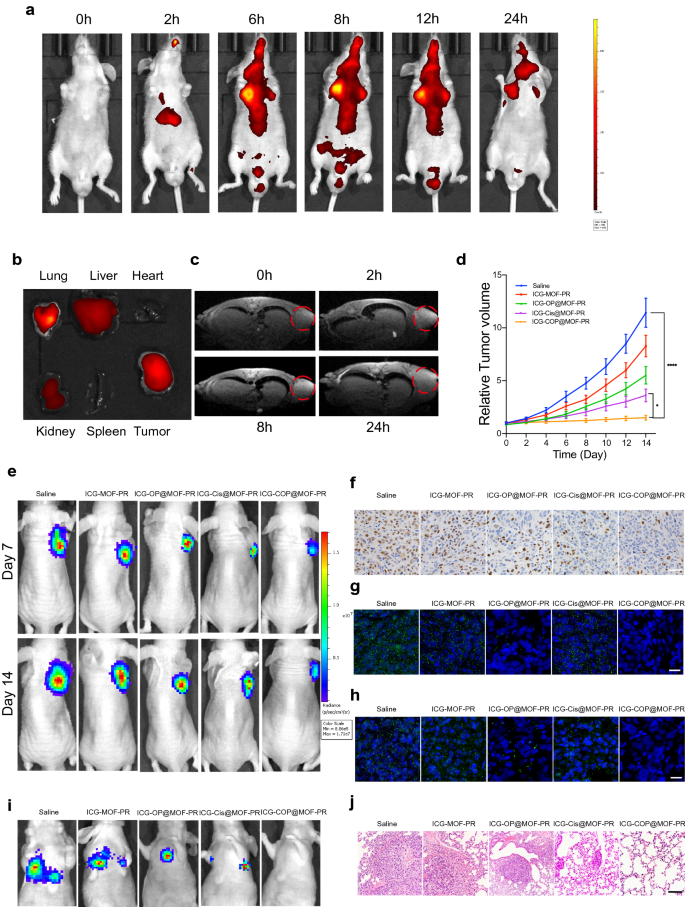
A nanoplatform that provides complementary anatomical and purposeful details about tumors by multimodal imaging is necessary for the exact detection and therapy of most cancers. We due to this fact evaluated the multimodal imaging capability of the multifunctional ICG-COP@MOF-PR nanoplatform in OCM1a-tumor-bearing mice. As a result of the fluorescence of ICG enabled its use as a desired agent for fluorescent imaging, we first evaluated the time-dependent biodistribution of ICG-COP@MOF-PR in OCM1a-tumor-bearing mice at predesigned time factors (0, 2, 6, 8, 12 and 24 h) after the intravenous tail injection of NPs (Fig. 8a). We noticed that fluorescence within the tumor area steadily elevated over time and that the strongest fluorescence sign appeared at 8 h post-injection of ICG-COP@MOF-PR. We additionally found that fluorescence remained within the tumor area till 24 h post-injection, which prompt that ICG-COP@MOF-PR may accumulate in tumors for an in depth interval. To raised examine the biodistribution of ICG-COP@MOF-PR, tumors and main organs (coronary heart, liver, spleen, lung, and kidney) have been collected after 24 h for ex vivo fluorescence imaging. As proven in Fig. 8b, the fluorescence sign was nonetheless current within the tumor tissue with partial liver, kidney and lung accumulation, which additional confirmed the lengthy retention interval of NPs in vivo. Notably, the fluorescence imaging functionality of ICG-COP@MOF-PR is useful to the real-time monitoring of its real-time in vivo distribution.
Pharmacokinetics and focusing on impact and tumor suppression results of intravenously injected NPs in vivo. a In vivo real-time bioluminescence imaging of UM tumor-bearing mice at completely different time factors after administration of ICG-COP@MOF-PR. b ICG fluorescent intensities from ex vivo imaging of the key organs, and the tumors 24 h after injecting ICG-COP@MOF-PR. c T1-weighted MRI after intravenous injection with ICG-COP@MOF-PR at predesigned time factors. d, e The tumor development curves and bioluminescence imaging confirmed UM development with completely different remedies in vivo. f Immunohistochemistry of Ki-67 of UM tumor samples with completely different remedies. Scale bar, 50 μm. g RNA in situ hybridization of lncRNA OUM1 (inexperienced) in tumor samples with completely different remedies. Scale bar, 20 μm. h RNA in situ hybridization of PTPRZ1 (inexperienced) in tumor samples with completely different remedies. Scale bar, 20 μm. i Bioluminescence imaging of M2b induced pulmonary metastasis fashions. j HE staining of metastasis nodes within the lung. Scale bar, 200 μm
Within the mildly acidic tumor microenvironment, the MOF in our nanodelivery system could be degraded, releasing Mn2+ and loaded medicine together with cisplatin and siRNAs. The launched Mn2+ not solely facilitates CDT however will even impacts the longitudinal relaxivity and MRI distinction impact, rendering the NPs a distinction agent for MRI [42]. As proven in Fig. 8c, ICG-COP@MOF-PR displayed an apparent and time-dependent T1-weighted enhancement in tumor tissues. Collectively, these knowledge verified that ICG-COP@MOF-PR might be utilized as a secure versatile agent for fluorescence/MRI dual-modal imaging with excessive distinction, to exactly monitor their tumor accumulation and to supply extra details about tumors from a extra correct and broader spectrum.
Focused siRNA interference-mediated remedy in synergy with CDT and enhanced cisplatin remedy suppressed UM tumor development and pulmonary metastasis
To guage the therapeutic impact of NPs in vivo, a subcutaneous xenograft mannequin constructed by way of subcutaneous injection of OCM1a cells and a pulmonary metastasis mannequin induced by means of the intravenous tail injection of extremely invasive UM cells, MUM2b cells have been established. OCM1a-tumor-bearing BALB/c nude mice have been randomly divided into 5 teams after which have been handled with saline, ICG-MOF-PR, ICG-OP@MOF-PR, ICG-Cis@MOF-PR or ICG-COP@MOF-PR. The tumor sizes have been measured each two days. After 14 days of therapy, vital inhibition and dimension discount have been noticed in tumors handled with NPs (Fig. 8d). Notably, ICG-COP@MOF-PR confirmed superiority over ICG-Cis@MOF-PR in tumor inhibition and displayed the most effective tumor inhibition effectivity in any respect the investigated time factors. Luciferase photographs of tumor-bearing mice at day 7 and day 14 additionally demonstrated the tumor inhibition impact of NPs (Fig. 8e). Tissue sections have been ready from tumor samples at 14 days after varied remedies, and the expression ranges of Ki-67 have been evaluated by IHC staining. The remedies with ICG-MOF-PR, ICG-OP@MOF-PR, ICG-Cis@MOF-PR and ICG-COP@MOF-PR induced downregulation of Ki-67 expression, and amongst these remedies, ICG-COP@MOF-PR exerted essentially the most vital suppression impact (Fig. 8f). RNA in situ hybridization of the lncRNAs OUM1 and PTPRZ1 was additionally carried out with the abovementioned tumor samples to research the expression of the lncRNAs OUM1 and PTPRZ1 (Fig. 8g and h). The sharp lower in OUM1 and PTPRZ1 expression after ICG-COP@MOF-PR therapy demonstrated a excessive knockdown effectivity. This discovering additional confirmed that siOUM1 and siPTPRZ1 might be efficiently loaded and transported into tumor cells and that OUM1 and PTPRZ1 have been knocked down.
A pulmonary metastasis mannequin was additionally evaluated by bioluminescence imaging and histopathological commentary at day 14. The bioluminescence imaging method is taken into account sufficiently delicate and is ready to detect as few as 100 tumor cells in vitro, and thus, tiny metastases that can’t be tracked by the bare eye could be captured [43]. As proven in Fig. 8i, all of the mice within the saline group exhibited apparent metastases within the lung, whereas pulmonary metastasis was inhibited after therapy with NPs. In sharp distinction, pulmonary metastasis was considerably inhibited by therapy with ICG-COP@MOF with no detectable pulmonary metastases (Fig. 8i). Histologic micrographs (Fig. 8j) revealed the identical outcomes.
Taken collectively, the subcutaneous xenograft mannequin and pulmonary metastasis mannequin all prompt the prevalence of ICG-COP@MOF-PR in inhibiting UM proliferation and metastasis. In our examine, the discharge of cisplatin, siOUM1 and siPTPRZ1 exhibited ROS- and acid-sensitive traits, which signifies that the ROS technology in flip promoted the discharge of the abovementioned medicine. When ICG-COP@MOF-PR was taken up by UM cells, cisplatin, siOUM1 and siPTPRZ1 have been launched. Subsequently, OUM1 and PTPRZ1 might be selectively knocked down and protein tyrosine phosphorylation within the UM microenvironment was interrupted, resulting in inhibition of proliferation and metastasis of UM. As well as, the knockdown of OUM1 and PTPRZ1 improved the sensitivity of UM cells to chemical medicine, enhancing the impact of cisplatin and additional inhibiting UM proliferation and metastasis.